Dynamic species distribution models of Antarctic blue whales in the Weddell Sea using visual sighting and passive acoustic monitoring data
Aim: Species distribution models (SDMs) are essential tools in ecology and conservation. However, the scarcity of visual sightings of marine mammals in remote polar areas hinders the effective application of SDMs there. Passive acoustic monitoring (PAM) data provide year-round information and overcome foul weather limitations faced by visual surveys. However, the use of PAM data in SDMs has been sparse so far. Here, we use PAM-based SDMs to investigate the spatiotemporal distribution of the critically endangered Antarctic blue whale in the Weddell Sea.
Location: The Weddell Sea
Methods: We used presence-only dynamic SDMs employing visual sightings and PAM detections in independent models. We compared the two independent models with a third combined model that integrated both visual and PAM data, aiming at leveraging the advantages of each data type: the extensive spatial extent of visual data and the broader temporal/environmental range of PAM data.
Results: Visual and PAM data prove complementary, as indicated by a low spatial overlap between daily predictions and the low predictability of each model at detections of other data types. Combined data models reproduced suitable habitats as given by both independent models. Visual data models indicate areas close to the sea ice edge (SIE) and with low-to-moderate sea ice concentrations (SIC) as suitable, while PAM data models identified suitable habitats at a broader range of distances to SIE and relatively higher SIC.
Main conclusions: The results demonstrate the potential of PAM data to predict year-round marine mammal habitat suitability at large spatial scales. We provide reasons for discrepancies between SDMs based on either data type and give methodological recommendations on using PAM data in SDMs. Combining visual and PAM data in future SDMs is promising for studying vocalized animals, particularly when using recent advances in integrated distribution modeling methods.
Location The Weddell Sea.
Methods We used presence-only dynamic SDMs employing visual sightings and PAM detections in independent models. We compared the two independent models with a third combined model that integrated both visual and PAM data, aiming at leveraging the advantages of each data type: the extensive spatial extent of visual data and the broader temporal/environmental range of PAM data.
Results Visual and PAM data prove complementary, as indicated by a low spatial overlap between daily predictions and the low predictability of each model at detections of other data types. Combined data models reproduced suitable habitats as given by both independent models. Visual data models indicate areas close to the sea ice edge (SIE) and with low-to-moderate sea ice concentrations (SIC) as suitable, while PAM data models identified suitable habitats at a broader range of distances to SIE and relatively higher SIC.
Main Conclusions The results demonstrate the potential of PAM data to predict year-round marine mammal habitat suitability at large spatial scales. We provide reasons for discrepancies between SDMs based on either data type and give methodological recommendations on using PAM data in SDMs. Combining visual and PAM data in future SDMs is promising for studying vocalized animals, particularly when using recent advances in integrated distribution modelling methods.
1 INTRODUCTION Information on the long-term spatiotemporal distribution of species is the keystone for effective management and conservation. However, obtaining sufficient sighting data over large spatial and temporal scales is challenging and costly for cetacean species, particularly for rare species (Burham et al., 2016; Williams et al., 2006). The distribution and abundance of cetacean populations are mainly estimated through shipboard visual line-transect surveys (Frasier et al., 2021; Kaschner et al., 2012). Although visual surveys may show good spatial coverage in some areas, they are resource-intensive, often limited to vessels operating exclusively within a nation’s EEZ (exclusive economic zone) and globally show large taxonomic, spatial and temporal gaps (Kaschner et al., 2012).
The visual detectability of cetaceans is low and subject to several limitations. Visual surveys depend on the availability of animals at the sea surface (Fleming et al., 2018; Frasier et al., 2021). Further, visual surveys are limited to daylight hours and fair weather conditions, affected by sea state, precipitation, fog and wind (Fleming et al., 2018; Miller et al., 2015; Rayment et al., 2017), which hampers their conduct during winter months, particularly in polar areas like the Southern Ocean (SO) (Burham et al., 2016; El-Gabbas et al., 2021a; Frasier et al., 2021). Extensive survey efforts are needed to obtain sufficient sightings for estimating species abundance and population trends, particularly for rare and elusive species (Kaschner et al., 2012). This is a challenging task even at regional scales due to the often prohibitive economic and logistic costs of undertaking surveys far offshore.
Alternative sampling techniques – for example, passive acoustic monitoring (PAM) and satellite tracking – can help improve our knowledge of cetacean distributions and migration patterns. PAM is a non-invasive method that detects underwater sounds of vocalizing species, providing consistent, seasonally unbiased data on the acoustic occurrence of sound-producing animals and consequently, contributes insights into their ecology, distribution patterns and behaviour (Fleming et al., 2018; Thomisch et al., 2016). PAM data can, therefore, overcome some challenges inherent to visual data. The collection of PAM data is less affected by bad weather conditions (i.e. weather impact on acoustic data quality occurs only indirectly through changing background noise levels) and the seasonal existence of sea ice, is omnidirectional, can cover larger detection ranges than visual sightings and can operate autonomously year-round equally well during day and night (Mellinger et al., 2007; Miller et al., 2015; Miller, Potts, et al., 2019). PAM data was recently shown as a valuable sampling technique to detect the year-round acoustic presence of rare or cryptic cetacean species over extended periods in areas and times not possible to perform using visual surveys (Fleming et al., 2018; Thomisch et al., 2016).
The Antarctic blue whale (ABW; Balaenoptera musculus intermedia) is classified as Critically Endangered by the International Union for the Conservation of Nature (IUCN) (Cooke, 2018) and is considered one of the most threatened baleen whale species in the SO. The SO, and in particular the Southern Boundary of the Antarctic Circumpolar Current (sbACC), is a known ecologically important region as high densities of krill are known to occur here, which forms the primary food resource for ABW (Atkinson et al., 2008; Tynan, 1998). The distribution of ABW is considered to be greatly influenced by prey availability, hence, the SO serves as an important (feeding) habitat for ABW (Branch, Stafford, et al., 2007; Thomisch et al., 2016). ABWs were once common in the SO, but more than 300,000 whales were hunted to near extinction in the Southern Hemisphere during the last century’s industrial whaling (Clapham & Baker, 2009; Cooke, 2018; Miller et al., 2015). Although their population tends to slowly increase again since the cessation of industrial whaling, some recent population estimates of about 3000 mature individuals suggest that they are still less than 3% of their pre-whaling abundance (Cooke, 2018) and it may take decades to reach half of their pre-exploitation abundance (Branch, 2008; Branch et al., 2004).
Knowledge of the year-round distribution and movement patterns of ABWs is crucial to their effective conservation and management (Shabangu et al., 2020). However, until the past two decades, knowledge of their distribution and abundance in the SO was limited to austral summer and mainly derived from historical catches and rare recent visual surveys (Calderan et al., 2020; Letsheleha et al., 2022; Širović et al., 2009), resulting in a poor understanding of their distribution, movement patterns and foraging behaviour (Miller et al., 2015; Miller, Potts, et al., 2019; Thomisch et al., 2016). Recent ABW visual sightings are highly limited by the subspecies’ scarcity and low encounter rate, their wide circum-Antarctic distribution, with associated seasonal sea ice cover and financial and logistic restrictions to surveys (Miller et al., 2015; Miller, Potts, et al., 2019; Shabangu et al., 2020; Thomisch et al., 2016). Year-round, adult male ABWs produce repetitive stereotypic low-frequency high-intensity calls (known as Z-calls) that propagate over several hundreds of kilometres (Miller et al., 2015; Širović et al., 2007), resulting in much larger detection ranges than attainable by visual sightings. In the Weddell Sea study area, the majority of ABW vocalizations (95th percentile) have been estimated to be emitted within 100 km of the respective recorders when assuming spherical spreading (Thomisch et al., 2016). During the last two decades, PAM has been increasingly used to study ABWs’ year-round distribution and acoustic behaviour, particularly in the highly remote SO (Leroy et al., 2016; Thomisch et al., 2016).
Species distribution models (SDMs) have become standard tools for estimating species’ habitat suitability (HS) and identifying factors affecting the distribution of the species (Guisan et al., 2017). SDMs on marine species are less frequent compared to terrestrial species; however, recent years have shown an increasing interest in using SDMs in the marine realm (Robinson et al., 2011), particularly when only presence data are available (Bombosch et al., 2014; El-Gabbas et al., 2021a, 2021b; Smith et al., 2020). Most cetacean SDMs use visual sightings, although the use of PAM data is currently increasing (Brookes et al., 2013; Fleming et al., 2018).
The main objective of this study is to explore the strengths and limits of modelling the spatiotemporal distribution of ABWs in the Weddell Sea using presence-only dynamic SDMs with PAM data. In dynamic SDMs, species detections are spatiotemporally matched with contemporaneous environmental conditions, allowing for predicting habitat suitability in time and space (see El-Gabbas et al., 2021a for more details on the difference between static and dynamic SDMs). Previous ABWs’ SDM studies in the SO have mainly used visual sightings (e.g., El-Gabbas et al., 2021a, 2021b). PAM data were frequently used to study the seasonal distribution patterns, acoustic behaviour and the relationship between ABW calls and environmental conditions (Shabangu et al., 2017; Širović & Hildebrand, 2011; Thomisch et al., 2016), yet not to make spatiotemporal predictions over large spatial scales. This study, for the first time, used PAM data to model the year-round dynamic distribution of ABWs in the Weddell Sea. Our motivation is that PAM data are less temporally biased and show a higher detection probability and broader coverage of environmental conditions than visual sightings, with the potential to improve model predictions (Frasier et al., 2021). However, since the complementary visual and PAM data may reflect different behavioural states (Fleming et al., 2018; Rayment et al., 2017), we compared the results of PAM models to models that use visual sightings only or a combination of visual and PAM datasets. We further determined where and when the daily predicted HS of the three model types agree or disagree.
2 METHODS 2.1 ABWs daily detections The study area encompasses the Weddell Sea (Figure 1) and the northern part of the West Antarctic Peninsula (WAP) (65° W–30° E). Latitudinally, it extends from the climatological mean of the Polar Front (Orsi et al., 1995) to the Antarctic ice shelf edge. ABW visual sightings from the SO were collated from different data sources and only quality-controlled sightings from within the study area that temporally match the availability of environmental predictors were considered (2003–2019). See Appendix S1 for the list of data sources and El-Gabbas et al. (2021a, 2021b) for more details on visual sighting data. In total, 132 distinct sightings were used in the models. The spatiotemporal distribution of sightings is shown in Figure 1 and Figure S1.
Details are in the caption following the image FIGURE 1 Open in figure viewer PowerPoint Map of the study area, the Weddell Sea (yellow-marked area). Blue triangles represent Antarctic blue whale visual sightings (2003–2019). Red squares show the location of the five acoustic recorders, with numbers representing the station ID (see Table 1). Numbers in black circles represent location names as used in the text. The temporal distribution of visual and acoustic presence-only data is shown in Figure 2 and Figure S1. PAM data were recorded by ten moored devices at five sites in the Weddell Sea and along the Greenwich meridian between March 2008 and November 2013 (59°–69° S and 0°–27° W; see Figures 1 and 2 and Figures S1 and S2; Table 1). The recording devices were part of the oceanographic deep-sea moorings of the Hybrid Antarctic Float Observation System (HAFOS; Rettig et al., 2013; Thomisch et al., 2016). Acoustic recorders of type Sono.Vault (manufactured by develogic GmbH, Hamburg, Germany; Rettig et al., 2013) or Autonomous Underwater Recorder for Acoustic Listening (AURAL; Model 2, manufactured by Multi-Electronique (MTE) Inc., Rimouski, Quebec, Canada; Simard et al., 2008) were used. Detailed information on the recording parameters of the passive acoustic recorders is shown in Table 1.
Details are in the caption following the image FIGURE 2 Open in figure viewer PowerPoint Schematic view of the Antarctic blue whale PAM data. Orange areas represent operation periods. We used an RL ≥ 109 dB threshold to determine daily acoustic detections. Blue symbols represent days with acoustic presences, grey symbols for days without acoustic data (e.g., due to device failure) and red symbols for days with no acoustic presences (i.e., maximum daily RL < 109 dB). More details on the recorders are shown in Table 1 and Figure 1. TABLE 1. Locations and recording parameters of passive acoustic recorders deployed within the Hybrid Antarctic Float Observation System (HAFOS) array in the Weddell Sea. Station ID Recorder ID Latitude Longitude Deployment period Deployment depth [m] Sampling freq. [kHz] Sampling scheme ([min]/[min]) Operational period [months] # daily acoustic presences # filtered acoustic presences 1 AWI209-06 AU0086 66° 36.70′ S 27° 07.31′ W 12/2010–01/2013 207 32.77 4.5/180 24 63 31 2 AWI227-11 SV0002 59° 03.02′ S 00° 06.63′ E 12/2010–12/2012 1007 5.33 Continuous 8 82 95 AWI227-12 SV1025 59° 02.63′ S 00° 04.92′ E 12/2012–12/2014 1020 5.33 Continuous 7 105 3 AWI229-09 SV1000 63° 59.56′ S 00° 02.65′ W 12/2010–12/2012 969 5.33 Continuous 6 47 70 AWI229-10 SV1010 63° 59.66′ S 00° 02.65′ W 12/2012–12/2014 969 5.33 Continuous 8 55 4 AWI230-06 AU0085 66° 01.13′ S 00° 04.77′ E 03/2008–12/2010 189 32.77 5/240 34 19 120 AWI230-07 SV1001 66° 01.90′ S 00° 03.25′ E 12/2010–12/2012 934 5.33 Continuous 21 91 AWI230-08 SV1009 66° 02.12′ S 00° 02.98′ E 12/2012–12/2014 949 5.33 Continuous 9 56 5 AWI232-09 AU0086 68° 59.74′ S 00° 00.18′ E 03/2008–12/2010 206 32.77 5/240 34 38 53 AWI232-11 SV1011 68° 59.86′ S 00° 06.51′ W 12/2012–12/2014 958 5.33 Continuous 11 53 Total: 609 Total: 369 Note: Sampling schemes are listed in terms of sampling duration [min] per sampling interval [min]. The location of the recorders is shown in Figure 1. The last column represents the number of daily acoustic presences after temporal filtering, see main text for more information. Like other blue whale subspecies, ABWs produce two call types, possibly reflecting different behavioural states (e.g., Barlow et al., 2023; Oleson, Calambokidis, et al., 2007; Oleson, Wiggins, & Hildebrand, 2007). Frequency-modulated downsweep calls (FM-calls; also known as D-calls) are assumed to be produced by all age and sex classes of blue whales (e.g., Lewis et al., 2018; Oleson, Calambokidis, et al., 2007), while Z-calls are likely produced only by adult ABW males (e.g., Oleson, Wiggins, & Hildebrand, 2007). Therefore, the acoustic presence data inferred from Z-calls may only represent a subset of the ABW population, possibly leading to underestimating their acoustic occurrence. However, although FM-calls may show a broader representation of ABWs distribution pattern and habitat suitability, estimations of source level and detection ranges of Antarctic blue whale FM-calls remain scarce to date. Recently, Miller et al. (2021) provided a source level estimation of 190 ± 5.5 dB for ABW FM-calls. In contrast to Z-calls, ABW FM-calls are highly variable in spectrographic shape and production. Furthermore, other baleen whale species are known to produce similar frequency-modulated calls, such as pygmy blue whales Balaenoptera musculus brevicauda (e.g., Gavrilov et al., 2011), Antarctic minke whales Balaenoptera bonaerensis (e.g., Dominello & Širović, 2016), fin whales Balaenoptera physalus (e.g., Thompson et al., 1992), or sei whales Balaenoptera borealis (e.g., Calderan et al., 2014), hence, complicating unambiguous (sub)species assignment for FM-calls. Automated detection methods have therefore rarely been applied to ABW FM-calls from the SO (but see Miller et al., 2023 for a very recent approach). Based on the uncertainty in source level and since detector development was beyond the scope of this study, we here used Z-calls to model the distribution of ABWs in the SO.
Z-calls, produced year-round by ABWs, are highly stereotypic, low-frequency vocalizations, typically composed of three units within the frequency range of 18–28 Hz (Ljungblad et al., 1998; Rankin et al., 2005). This study used the daily acoustic presence of ABWs based on previous automated detections of Z-call vocalizations as described in detail in Thomisch et al. (2016). ABWs Z-calls were detected by spectrogram cross-correlation using a pre-defined spectrogram template (e.g., Mellinger & Clark, 2000) in a frequency band from 17.5 to 29 Hz, accounting for inter- and intra-annual variations in the ABW Z-call frequency. The spectrogram correlation had an estimated average false detection rate of 1%. Received levels (RLs) were obtained for each detected Z-call, as explained in detail in the supplement of Thomisch et al. (2016): upper components of auto-detected Z-calls were extracted from bandpass filtered audio files (Butterworth filter, passband 25–29 Hz) and the received sound pressure level SPLRMS [dB re: 1 μPa] within the 25–29 Hz band of each Z-call event was determined. The 25–29 Hz frequency band features the loudest part of an ABW Z-call.
The low-frequency ABW vocalizations can be detected at large distances (up to hundreds of kilometres) (Širović et al., 2007; Thomisch et al., 2016). This likely results in a spatial mismatch between the actual position of calling animals and the recorders, which can affect the robustness of the models, particularly in the highly dynamic SO (El-Gabbas et al., 2021a). Therefore, we filtered ABW detections to only keep detections that originated from within a ~10 km radius from the recorders, that is, the typical grid cell size of the environmental predictors. Approximate distances between vocalizing ABWs and the respective recorder locations were estimated assuming a source level of 189 dB re: 1 μPa over 25–29 Hz (Širović et al., 2007) and a spherical transmission loss TL[dB] = 20log10(r), noticing that horizontal distances considered and water depth are of the same magnitude. Daily acoustic presences were estimated as days with at least one detection event within a ~10 km radius of the respective recording sites; that is, Z-calls with calculated received levels of ≥ 109 dB, considering a nominal TL of 80 dB.
In total, 609 daily detections were determined at the five recording sites (Table 1). The temporal distribution of acoustic presences is shown in Figure 2 and Figure S1. To avoid the effect of temporal non-independence (temporal autocorrelation) between consecutive daily acoustic presences on the model, we temporally filtered acoustic presence data from each recording site to a single acoustic presence event per 3-day interval (resulting in a total of 369 daily acoustic presences). At each recording site, we split the time series data into 3-day bins. Then, if more than 1 day with acoustic detections is recorded in a particular bin, we considered this bin as acoustic presence and assigned to this presence event the mean value of all predictors on days with detections. We calibrated exploratory models to see if the temporal filtering step has a noticeable effect on predicted HS. HS maps from models that used unfiltered and filtered data (Figure S3) lacked notable differences and temporally filtered data were therefore used in the final models.
2.2 Environmental data In previous models, we used a combination of static and dynamic predictors, which both affect the presence of top predator species and their prey (Stanton et al., 2012; Wyles et al., 2022), to model the distribution of ABWs in the SO using visual sighting data. The effect of static predictors (depth, distance to coast, slope and distance to 1000 m isobath) is shown in El-Gabbas et al. (2021a, 2021b). However, our PAM data were collected from five grid cells only, which inevitably renders the use of static predictors inappropriate (Rayment et al., 2017). Therefore, we confined the models in this study to dynamic predictors.
We used six daily dynamic predictors from 2003 to 2019. A detailed description of the predictors used is given in El-Gabbas et al. (2021a). Three of these predictors were extracted from daily sea ice data (Spreen et al., 2008), downloaded from https://seaice.uni-bremen.de/ at 6.25 km resolution: sea ice concentration (SIC), distance to the sea ice edge (SIE) and the lagged SIC variance throughout the 14 preceding days. Daily sea surface temperature (SST) was downloaded from JPL MUR MEaSUREs Project (2015) at 0.01° resolution. Daily sea surface height (SSH) data were obtained from Copernicus (https://copernicus.eu/) at 0.25° resolution. From SSH data, we used absolute dynamic topography to represent SSH, while we calculated the current speed from the zonal and meridian components of the absolute geostrophic velocity. All daily dynamic predictors were projected onto a 10 × 10 km2 equal-area grid and cropped to the study area.
2.3 Model fitting and evaluation We considered both visual and acoustic detections as presence-only data (see the Discussion section for the justification of considering PAM data as presence-only) and used the presence-only SDM algorithm Maxent (v3.4.4; Phillips et al., 2017). Daily (visual and acoustic) detections were spatiotemporally matched with dynamic predictors (Figure S4). Maxent requires, in addition to species detections, sufficiently sampled background information representing the environmental conditions in the study area (Renner et al., 2015). We used extensive background information from the SO, sampled daily from 2003 to 2019, using seasonally estimated research efforts in the SO as a probability weight to avoid spatial sampling bias in visual sightings on model performance and inference (El-Gabbas et al., 2021a; El-Gabbas & Dormann, 2018). In total, 11.7 million background locations from the study area were used to run the models.
To explore differences between models that employed ABWs’ visual and PAM detections, we calibrated three sets of models, different on which species data is used and how models were evaluated on cross-validation: visual data only (Modelvisual), PAM data only (ModelPAM) and a third model that uses both data types together (Modelcombined). In Modelcombined, both visual and PAM data were treated equally; that is, the daily presence data of either data type were pooled together and used as the response variable of the model.
We evaluated model performance using two methods. First, we implemented the standard ‘internal’ model evaluation using spatially independent testing data of the same data type. For Modelvisual, spatial blocks were used for cross-validation, using a block size of 25 cells (i.e., 250 × 250 km2) distributed into four cross-validation folds while maintaining a balanced number of sightings between folds (Figure S5). For ModelPAM, we used the site location (Figure 1) for cross-validation; that is, five-fold cross-validation. The distance between the five PAM sites ranges between 222 and 1621 km, sufficiently large to assume spatial independence between sites, in particular since we used acoustic presences only within ~10 km from detectors. For Modelcombined, we used both cross-validation structures to fit the models, that is, nine cross-validation folds, four for the visual part and five for the acoustic part of the data.
Second, to examine whether the complementary nature of visual and PAM data is also reflected in model outputs and predicted HS, we estimated the predictive ability of each model type at the presence locations of the other data type. We refer to this as the ‘external’ evaluation. For Modelvisual, we calculated testing AUC/TSS (see below) by comparing predicted values at the time and location of PAM detections versus a sample of background locations. Similarly, for ModelPAM, we compared predicted values at the time and location of visual observations versus a sample of background locations. It is expected that values for external model evaluation will be smaller than for internal evaluation because of discrepancies between both data types (more details in the Discussion section). However, our motivation for using external model evaluation here is to show the complementarity of both data types and to test the potential limitation of SDMs using visual sightings data to predict vocalizing individuals and vice versa.
Model performance was evaluated using two metrics: the area under the ROC curve (AUC) and true skill statistics (TSS). We used the threshold that maximizes the sum of testing sensitivity and specificity to calculate TSS (Liu et al., 2013). AUC values range between 0 and 1, while TSS values range between −1 and 1 (higher values represent better models for both metrics). Since the estimation of model performance can be sensitive to the number of testing presences or the prevalence of testing data (ratio between the number of testing presences and background locations) (Lobo et al., 2008; Sofaer et al., 2019), we maintained a test-data prevalence of 1:100 to ensure the validity of comparisons between models (El-Gabbas & Dormann, 2018). We repeated this step 1000 times to average stochastic effects, each with a different sample of background locations. We plotted the raw evaluation values distribution in the Appendix S1 and reported mean values in the main text.
For each of the three model types, we predicted mean daily HS between 2003 and 2019, weighted by the respective mean cross-validated testing AUC. In the Appendix S1, we present daily HS maps on the 15th day of each month in 2013 (Figure S6). Daily HS maps were converted into animated videos to show how HS changes over time. To find out how the daily HS of the three model types overlap spatially, we estimated their daily congruence using three metrics: Schoener’s D, Warren’s I (Warren et al., 2008) and Pearson’s correlation coefficient. Further, we overlay monthly suitable habitats using the three model types (more information on how monthly suitable habitats were determined is given in the figure caption to Figure S7). We compare the results of the three models to the dynamic models of El-Gabbas et al. (2021a), which used circumpolar ABWs’ visual sightings and both static and dynamic predictors. Further, we extracted the predictors’ permutation importance and single/marginal response curves for each model type. Marginal response curves show how predicted HS changes as each environmental variable is varied while other variables are fixed at their average sample value. As marginal response curves can be sensitive to the value at which other variables are fixed (El-Gabbas et al., 2021b), we also show the pairwise interactive effect of predictors on the predicted HS.
3 RESULTS The vast majority of ABW visual sightings were made between December to mid-February, with only a single sighting in late February, two in March, one in April and two in November. Acoustic data showed a much broader temporal coverage of ABWs detections (Figure S1). There are gaps in acoustic data from August to mid-December at the two northmost sites (AWI227 and AWI229) due to instrument failure (Figures 1 and 2 and Figures S1 and S2). Most of the ABW acoustic detections at the southernmost recording site (AWI232) were from January to March, with only sporadic detections in other months, while at the Weddell Basin site (AWI209), detections were made mainly from the end of February to mid-April. Using visual and PAM data has resulted in complementary patterns of habitat suitability and low spatial overlap between their daily predictions, while ModelPAM and Modelcombined highly overlapped (Figures 3 and 4 and Figure S11). Internal model evaluation of the three models (cross-validation using spatially independent data of the same type) was generally high (mean testing AUC ranged from 0.77 to 0.92; Table 2 and Figure S12), with Modelvisual having higher values than the other two model types. Using the northernmost recording site (AWI227; Figure 1) to cross-validate ModelPAM resulted in much lower evaluation values than for the other sites (Figure S12b). Contrastingly, external model evaluation (i.e., using one data type to evaluate models run on the other data type) was lower for both model types: 0.64 ± 0.02 for ModelPAM (using PAM data to run the models, visual data for evaluation) and 0.62 ± 0.03 for Modelvisual (using visual data to run those models, PAM data for evaluation; Table 2 and Figure S13).
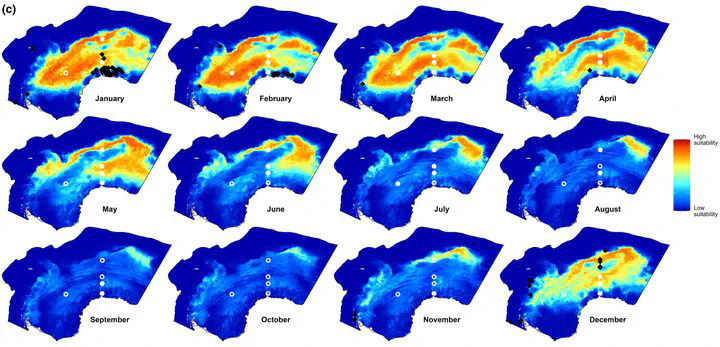
Details are in the caption following the image FIGURE 3 Open in figure viewer PowerPoint Monthly summary of predicted habitat suitability of Antarctic blue whales of the four model types implemented. Each map shows the 90th quantile of daily predicted habitat suitability in the respective month and model type from 2003 to 2019. White circles indicate the location of PAM recorders. These circles are filled with white color if there is any acoustic presence in the respective month. Black symbols show visual sightings in the respective month. Biweekly summary maps are shown in Figure S8. Examples of daily habitat suitability maps on the 15th day of each month in 2013 are shown in Figure S6. (a) Modelvisual (only dynamic predictors; this study). (b) Previous visual data models: Circumpolar models, with both static and dynamic predictors (El-Gabbas et al. 2021a). (c) ModelPAM. (d) Modelcombined. Details are in the caption following the image FIGURE 4 Open in figure viewer PowerPoint Spatial overlap (Warren’s I metric; Warren et al., 2008) between daily mean predicted habitat suitability of the three model types. Blue dots represent daily overlap values between Modelvisual & ModelPAM; red for Modelvisual & Modelcombined; and grey for ModelPAM & Modelcombined. Black lines show the mean overlap value of the respective model combination for each calendar day. Warren’s I value ranges from zero for no overlap to one for complete overlap. For similar results using Schoener’s D metric and Pearson’s correlation coefficient, see Figure S11. TABLE 2. Model evaluation results. Visual-data models PAM-data models Combined-data models Cross-validation ExternalPAM Cross-validation ExternalVisual Cross-validation Testing AUC 0.92 ± 0.02 0.62 ± 0.03 0.78 ± 0.15 0.64 ± 0.02 0.77 ± 0.11 Testing TSS 0.75 ± 0.02 0.22 ± 0.1 0.52 ± 0.29 0.34 ± 0.03 0.53 ± 0.12 Note: This table shows values of mean testing AUC and TSS, either on cross-validation (internal model evaluation) or using one data type to evaluate models that used another (external model evaluation; ExternalPAM and ExternalVisual). AUC values range between 0 and 1, while TSS values range between −1 and 1. Histograms for raw evaluation data are shown in Figures S12 and S13. Animated videos for daily HS of the three model types are available in El-Gabbas et al. (2023). We mainly refer to these daily maps when describing the year-round patterns of ABW’s HS. However, for the readers’ convenience, the monthly and biweekly patterns of HS are shown in Figure 3 and Figure S8. Generally, Modelvisual predicted high HS near the SIE. A moderate HS was predicted at northern parts of the study area (ca. 55–62° S) between June to August, peaking thereafter between September and November. From the end of November to February, HS intensifies and gradually shifts southwards, following the SIE retreat. In January–March, HS reaches its southernmost limit, being confined near the Antarctic coast, particularly in mid-February (30° W–30° E). From March to June, models generally predicted low HS, but locations near the SIE sporadically had moderate HS. The area off the Antarctic Peninsula (AP) eastwards over the South Scotia Ridge to the South Sandwich Islands was predicted with moderate-to-high HS near year-round, particularly from September to May.
ModelPAM predicted a broader pattern of HS. HS extended southwards between November and mid-March and then retracted northwards from April to July. Although this generally follows changes in the location of the SIE, high HS areas were not confined to near the SIE: they extend up to tens of kilometres on both sides and hundreds of kilometres north of the SIE in open water. ModelPAM predicted low HS in areas close to the Antarctic coast. From August to October, moderate-to-high HS was confined to a small patch (20° E 57° S, almost halfway between Antarctica and Africa). The area from the AP eastwards to the South Sandwich Islands was predicted with low-to-moderate HS, except sporadically between mid-November and December. In this area, HS is generally lower than Modelvisual.
Modelcombined showed significantly broader and more intense HS than the other two model types, covering high HS areas of both Modelvisual and ModelPAM. Generally, the three models predicted year-round low HS at the north of the sbACC and in the southern part of the Weddell Sea, except off the Brunt Ice Shelf (Figures 1 and 3) near 30° W in January and February, even in areas located south of the SIE.
For all models, the most important predictors were SSH, SST and distance to the SIE; however, some variation exists between model types (Figure 5). Response curves are shown in Figure 6 and Figures S9 and S10. Models predicted low HS at high SIC values; however, using PAM data in ModelPAM and Modelcombined indicated higher suitability at high SIC values as compared to Modelvisual. Areas close to the SIE were predicted with high suitability in all models; however, Modelvisual and Modelcombined show high suitability closer to the SIE as compared to ModelPAM. Yet, using PAM data identified high HS at two other values of distance to the SIE: at ca. 1000 km north and south of the SIE. Lagged sea ice variance showed no pronounced effect, except for ModelPAM, which predicted low HS at high values. Peak HS was predicted at SST values from −1.5°C to 1.5°C; −1.4 to −1.2 m for SSH.
Details are in the caption following the image FIGURE 5 Open in figure viewer PowerPoint Permutation importance of predictors used to model the distribution of Antarctic blue whales. Bars represent the mean predictor importance on cross-validation, with different colours for each model type. Red error bars show the standard deviation of the importance of the respective model. The ‘×’ symbols represent cross-validated raw permutation importance. Details are in the caption following the image FIGURE 6 Open in figure viewer PowerPoint Marginal response curves for Antarctic blue whale models. To create these plots, all predictors were used to run the models and then each predictor’s response curve was drawn by fixing all other predictors at their mean value at training presences. Lines and shaded areas represent the mean and standard deviation of response curves on cross-validation, with a different colour for each model type. In each plot, the top rugs show the spatiotemporally matched conditions at species detections (visual sighting in blue, PAM data in red), while the bottom green rug shows values at a sample of background locations from the Weddell Sea (10% of background information used to run the models). Environmental values at PAM detections, grouped by the location of the acoustic stations, are shown in Figure S4. See also Figure S9 for single variable response curves and Figure S10 for summary values of predicted habitat suitability in the environmental space of pairwise predictors. 4 DISCUSSION 4.1 Spatiotemporal distribution of ABWs in the Weddell Sea This study used presence-only SDMs to predict the spatiotemporal distribution of ABWs in the Weddell Sea, using both visual and acoustic data. Our results show that this is a promising approach to improve our understanding and direct future research for elusive species such as ABWs. Our models predicted a low-to-moderate HS for ABWs in the northern part of the study area between June and September. From November to March, areas with high HS extended southwards following the sea ice retreat. From April to July, the area of high ABW HS retracted northwards in response to heavy sea ice formation. Although this overall temporal pattern of shifting HS holds for ModelPAM and Modelvisual, areas identified with high HS were different using either dataset.
For Modelvisual, the area from the AP eastwards to the South Sandwich Islands has a near year-round moderate-to-high HS. This area overlaps with the location of the sbACC, southern Antarctic Circumpolar Current Front, the annual location of the SIE and (partially with) the continental shelf break (Figures 1 and 3). These oceanographic and bathymetric features are characterized by high seasonal primary productivity and Antarctic krill abundance (Euphausia superba, the main prey for ABWs), representing important habitats for many marine mammal and seabird species (Cuzin-Roudy et al., 2014; Hofmann et al., 2004; Miller, Calderan, et al., 2019; Siegel, 2005; Thomas & Green, 1988; Tynan, 1998). The continental shelf break is an important predictor for ABWs in the SO, with high ABW HS and large numbers of Z-calls close to it (El-Gabbas et al., 2021a; Miller, Calderan, et al., 2019). This highly suitable area, up to South Georgia, is considered an important ABW feeding area during summer (Wiedenmann et al., 2011) and was the primary location of ABW commercial whaling (Branch, Stafford, et al., 2007; Kemp & Bennett, 1932; Leaper & Miller, 2011; Risting, 1928). Historic catch data reported a year-round presence of ABWs off South Georgia (Harmer, 1931; Hinton, 1915; Risting, 1928), but locally, ABWs have reached near extinction from the area during the whaling era. However, recent studies suggest that ABWs have started to return to South Georgia, with some recent sightings from the area (Calderan et al., 2020). Although our models did not predict high HS close to South Georgia, probably due to the lack of recent sightings from this area, our results suggest that the area off the AP to the south of South Georgia is important for ABWs. More sighting (or PAM) effort off South Georgia is needed to improve future models’ predictability in this area.
Furthermore, other studies showed a year-round acoustic presence of ABWs from the WAP area (Širović et al., 2004), while ModelPAM did not predict a consistently high HS for this area. This contrasting finding can be due to the lack of acoustic data from the WAP area in this study. Modelvisual predicted high HS near the Antarctic coast between December and March (30° W–30° E), highly overlapping with the location of the continental shelf break, coastal polynyas in December and the SIE during those months (Figure 1 and Figures S14 and S15). In previous studies, distance to the coast was shown to be a significant predictor for the distribution of ABWs in the SO, with high HS close to the Antarctic coast (El-Gabbas et al., 2021a; Shabangu et al., 2017). We excluded static predictors (e.g., distance to coast and distance to continental shelf break) in this study to allow valid comparisons between ModelPAM and Modelvisual, which led to a broadening of HS (Figure 3a,b) (El-Gabbas et al., 2021a). The predictions from both models show high spatial overlap in HS (Figure S16). ModelPAM predicted a broader HS pattern than Modelvisual, overlapping with the location of the Weddell Gyre. The Weddell Gyre supports the presence of large krill populations in much higher concentrations than in other sectors of the SO (Laws, 1985). Interestingly, in the Atlantic sector of the SO, the sbACC was predicted to be the northern limit of high HS area for all models. This strongly agrees with information from visual and catch data (Branch, Stafford, et al., 2007; Calderan et al., 2020; Tynan, 1998) as well as PAM studies (Shabangu et al., 2017; Širović et al., 2009). The sbACC has a critical ecological function in the SO ecosystem, influencing the distribution of many cetacean species, with areas close to it being seasonally associated with high primary production and krill abundance (Matsuoka et al., 2003; Tynan, 1998). Nevertheless, our (visual and acoustic) detections are limited to the south of the sbACC, resulting in low HS north of it. Available environmental data show that the area north of the sbACC is almost always ice-free (Figure S15) and exhibits environmental conditions different from those occurring at acoustic recorder sites (Figure S17). While it is plausible to assume ABWs’ presence north of the sbACC during their migration, the lack of ABW detections associated with environmental conditions similar to those occurring in this area is likely to have resulted in low HS there.
Patterns of HS in this study support the general migration paradigm of ABWs between the highly productive high-latitude feeding grounds during austral summer and the low-latitude breeding grounds during austral winter (Branch, Stafford, et al., 2007; Miller et al., 2014). However, the near year-round acoustic presence (Figure 2) and predicted high HS in some areas (e.g., WAP area eastwards, Figure 3) also support that migration may not be obligatory. ABW migration is likely to include either partial or differential migration or a mixture of both, instead of an obligate complete migration of all individuals (Thomisch, 2017).
Although historical catches extended to areas north of the SIE, recent visual encounters, after the near complete extirpation of ABWs during commercial whaling, were confined mainly close to the SIE (Branch et al., 2004; Branch, Stafford, et al., 2007; Horwood, 1986; Leaper & Miller, 2011; Risting, 1928). It is well recognized that areas near the SIE are highly productive and rich in krill (Miller, Potts, et al., 2019; Paarman et al., 2021). Modelvisual predicted high HS near the SIE following sea ice expansion or retreat. Visual sightings of cetaceans from the SO are largely limited to near the SIE due to ship navigational constraints (Scheidat et al., 2011) posed by the presence of sea ice farther south. This spatial bias, as evident in the distribution of sightings in environmental space (Figure S4d), restricts Modelvisual’s ability to predict high HS south of the SIE and potentially reflects incomplete (truncated) species–environment relationships. In contrast, ABWs’ PAM data show a broader range of environmental conditions, from south of the SIE and at higher values of SIC (Figure S4a,b). In addition to near the SIE, ModelPAM predicted high HS also at large distances on both sides of the SIE (Figure 6).
Modelvisual and ModelPAM showed a negative relationship between ABWs’ HS and SIC (Figure 6). This conforms to information from previous studies (El-Gabbas et al., 2021a, 2021b; Širović et al., 2004; Širović & Hildebrand, 2011). However, ABWs were detected acoustically at high SIC values, which is also reflected in the relatively higher HS at high SIC values (Figures 6 and S4; Thomisch et al., 2016). ABWs appear to venture into the pack ice during summer and have been observed in areas with heavy sea ice cover (Branch, 2007; Branch et al., 2004; Double et al., 2015; Ruud, 1956; Slijper, 1962). In line with these observations, our models suggest that ABWs show some degree of tolerance to sea ice. Local coastal polynyas south of the SIE may support baleen whale species with food and access to the sea surface for breathing, enabling them to overwinter in the SO and occupy areas largely covered by heavy ice (Van Opzeeland et al., 2013). Our estimation of the SIE indicates the existence of recurrent polynyas off Brunt, Filchner and Ronne Ice Shelves from November to March; almost year-round off the AP, and off Maud Rise from September to December and in May and June (Figure S14). ModelPAM predicted high HS off the Maud Rise area from December to June (Figure 3). Upwelling, large swarms of krill and recurrent polynyas off Maud Rise back the ecological importance of this area as ABW habitat, also during winter (Paarman et al., 2021; Shabangu et al., 2020). Nevertheless, the actual occupation of these polynyas by the massive-bodied ABWs requires final confirmation.
Notwithstanding the evident effect of SIE and SIC on the predicted HS of ABWs, models nevertheless identified SST and SSH as the two most important predictors (Figure 5). Both predictors were important for ABWs call occurrence (Shabangu et al., 2017). Our models predicted a peak HS at SST values from −1.5°C to +1.5°C, although visual sightings were also made up to 3°C (Figure 6 and Figure S4d). This strongly agrees with other studies south of the Polar Front, using acoustic (Shabangu et al., 2017) and visual (Kasamatsu et al., 1988, 2000) data. At low SST (−2°C to −1.5°C), Modelvisual predicted much lower HS than ModelPAM, reflecting the unavailability of visual sightings at SST < −1.5°C. High HS areas (and the most of ABWs’ visual and acoustic detections) were predicted at SSH values between −1.5 and −1.1 m (Figure 6), which agrees with the results of Shabangu et al. (2017). The habitat preference of ABWs to this range of SSH may explain the low HS north of sbACC and off South Georgia which are characterized by SSH values > −1.1 m (Figure 3 and Figures S17 and S18). The location of eddies and fronts in the SO is strongly connected to specific values of SSH, which can highly affect primary production and the distribution and abundance of prey (Shabangu et al., 2017).
4.2 Differences between models based on visual versus acoustic data Our results showed that visual and PAM data show a complementary picture of the spatiotemporal distribution and habitat of ABWs: each data type has its pros and cons. This discrepancy was evident in the low spatial overlap between daily HS maps and some (albeit small) differences in the estimated environmental relationships (Figures 3, 5, 6). To quantitatively compare models that use either data type, it would be important to standardize research efforts, for example, by simultaneously collecting visual and acoustic data (e.g., using towed hydrophones) from the same platform (Dalpaz et al., 2021; Fleming et al., 2018; Miller et al., 2015). However, it is hard, if not impossible, to standardize long-term visual and PAM research efforts throughout the vast SO. Although differences between the outputs of both models can be due to differences in sample size and research efforts, our motivation for comparing Modelvisual and ModelPAM is to highlight differences between both data types, particularly in the remote SO. By exploring the role passive acoustic data can play in SDMs, we aim to improve our understanding of the distribution of ABWs.
Internal model evaluation via cross-validation (using spatially independent data of the same type) was generally high: mean testing AUC ranged from 0.77 to 0.92 (Table 2 and Figures S12 and S13). This reflects that models were reliable at predicting ABWs’ habitat suitability at spatially independent presence locations of the same data type. By contrast, external model evaluation (evaluating model performance using the other data type) was lower than internal evaluation for both data types (mean testing AUC ranged from 0.62 to 0.64; Table 2 and Figures S12 and S13). In other words, a model using one data type has a lower ability to predict high HS at the presence locations of the other data type. The overall lower external evaluation values of both model types indicate that both data types are complementary and may reflect different behavioural states of ABWs in the SO. This suggests that using either visual or PAM data alone may be insufficient to predict the spatiotemporal distribution of vocalizing baleen whales year-round.
Although visual data may show more extensive spatial coverage, the elusiveness, rarity, wide circumpolar distribution and low detectability of ABWs and the seasonal existence of heavy sea ice impede the efficient use of visual data to monitor ABWs’ year-round distribution (Shabangu et al., 2020; Thomisch et al., 2016). During the 32 years of IDCR-SOWER programmes in the SO (totalling 4112 days and 216,000 nm transect lines), visual sightings surveys resulted in only 216 individual encounters (Leroy et al., 2016; Miller et al., 2015). Further, distinguishing blue whale subspecies is difficult in the field: ABWs and pygmy blue whales (PBWs; B. m. brevicauda) look almost identical and it is difficult to distinguish the shorter PBWs from ABW during distant sightings (Branch, Abubaker, et al., 2007; Ichihara, 1966; Samaran et al., 2010; Torterotot et al., 2020). Admittedly, this may be more problematic at low latitudes, since PBWs are present mainly in the Indian Ocean and the western part of the south Pacific sectors of the SO, while at high latitudes (south of 52° S), PBWs constitute only <1% of blue whales (Branch, Abubaker, et al., 2007). However, in contrast to their similar appearance, both subspecies differ in their vocal behaviour, and the stereotypic Z-calls of ABWs can be easily detected, giving an advantage use of PAM to reliably detect and monitor ABWs distribution.
Acoustic data covers a broader range of environmental conditions compared to visual data (Figure S4) and their use in ModelPAM led to a broader pattern of HS. Nevertheless, ModelPAM did not consistently predict high HS in some areas predicted suitable by Modelvisual. Some visual sightings were observed in environmental conditions outside the range at acoustic detections (e.g., higher SST and SSH, Figure S17), which can explain the discrepancy between both model types. A possible reason is that our PAM sites are not evenly distributed in the study area but are located mainly along the Greenwich meridian. Available environmental data suggests that the area off the AP eastwards exhibits environmental conditions beyond the values at acoustic presences (for SST, SSH and current speed; Figures S17 and S18), which may also explain the low HS off South Georgia. Despite the added value of PAM versus visual data, as discussed above, the use of PAM data for HS modelling in general faces some caveats summarized later on.
In summary, differences in the patterns of predicted habitat suitability from models employing visual versus PAM data can be driven by multiple factors. The data used in the models is subject to different causes of biases and marine mammal behavioural states. For example, visual surveys strictly rely on the availability of animals at the sea surface during daylight hours and fair-weather conditions (often leading to spatiotemporal biases towards summer months and areas with little to no sea ice so ships can access). On the contrary, PAM can operate year-round for extended time periods, but is generally limited to a few recording sites. PAM data only represents calling individuals within the (species-specific) detection range of the site: here, Z-calls presumably represent adult male ABWs calling in specific behavioural states (see next section). Such differences between both data types are also reflected in differences in the amount of available data and values of environmental conditions in the n-dimensional space at presence data. For example, ABW PAM detections occur across a broader range of (higher) SIC and distance to SIC compared to visual data; see Figures S4 and S10 for a comparison of values for all predictors).
Such discrepancy between data types and model results should be considered with caution while interpreting patterns of habitat suitability. This is particularly important when studying rare and critically endangered taxa like the ABWs. Since there is no superior data type, we strongly recommend carefully comparing patterns of predicted habitat suitability using both visual and PAM data in isolation. Further, integrating visual and PAM data in a single SDM is advisable to overcome biases and provide a more holistic understanding of a species’ spatiotemporal distribution patterns.
4.3 Challenges of PAM-based SDMs PAM is presence-only data: Acoustic detections only provide information on the physical presence of calling individuals within the detection range of the acoustic device; that is, they cannot be used to infer the physical absence of baleen whales, as the animal can be present near the detector but not vocalizing (Letsheleha et al., 2022; Moore et al., 2011; Širović et al., 2009; Širović & Hildebrand, 2011) or vocalizing but outside the device’s detection range. For species that use echolocation for navigation and foraging (e.g., odontocetes), acoustic detections can be used as a much more reliable indicator of their physical presence since the production of these signals are produced by all age classes and sexes throughout the year (Soldevilla et al., 2010). However, even sperm whales were observed to remain silent for hours while visually observed on the sea surface (Dalpaz et al., 2021). For species that use sound in behavioural contexts that are more season-specific (e.g., songs produced by baleen whales), the detectability of the physical presence of these animals is inevitably imperfect (Rayment et al., 2017). Further aspects affect acoustic detectability; for example, call production and propagation conditions can vary in time and space, the ability to reliably detect acoustic presence can be subject to changing background noise levels and vocal behaviour can be highly dependent on species’ behavioural contexts. For the target species of this study, Širović et al. (2009) reported the absence of ABW calls at four circumantarctic locations in December and January, although historical catches and recent sightings peak during this period, probably implying that, although ABW Z-calls are produced year-round, calls may be produced in certain behavioural states (e.g., swimming, but silent while feeding) (Širović et al., 2009; Širović & Hildebrand, 2011). Z-calls are presumed to be produced only by adult males for reproductive display (Attard et al., 2016); therefore, the physical presence of females and juvenile males cannot be detected by Z-calls. Furthermore, determination of the number of calling animals, necessary for abundance estimations, is challenging based on PAM data; that is, whether the detected high vocalizations represent single/few animals calling a lot or many animals calling sporadically (Dalpaz et al., 2021; Rayment et al., 2017; Širović & Hildebrand, 2011). Hence, PAM data appears compatible only with presence-only SDMs. Species identification: For the majority of marine mammal species, the vocal repertoire has been described (Rankin et al., 2005) and many feature unique (with regard to species) and comprehensive (with regard to seasonal and contextual coverage) vocalizations. Nevertheless, for some species, not all calls are equally suitable to be included in SDMs. Some call types may occur in rare behavioural contexts (Klinck et al., 2010) only, or may have strong similarities to the calls produced by other species, such as the FM call in baleen whale species (Rankin et al., 2005; Thomisch, 2017). Here, we use Z-calls, which can be easily detected automatically and unambiguously assigned to ABWs. However, male and female ABWs as well as fin and humpback (Megaptera novaeangliae) whales also produce low-frequency FM calls for social interactions and feeding (Baumgartner & Fratantoni, 2008; Darling, 2015; Dominello & Širović, 2016; Gavrilov et al., 2011; Rankin et al., 2005; Thomisch, 2017). Therefore, target calls for constructing species-specific SDMs need to be selected with care so that detections are not contaminated by presence information from other species. Environmental mismatching: In this study, we used a pre-defined sound pressure threshold to exclude detections for animals calling at larger distances from the recording sites. Although this step has resulted in discarding many days as acoustic presences (i.e., yielding a smaller sample size representing a more confined range of environmental conditions), this step was essential to avoid a spatial mismatch between the location of the calling animals and the highly dynamic environmental conditions in the study area. For example, using a RL threshold of 100 dB (~28 km radius) led to matching ABWs’ daily detections to much higher values of the SIC and from locations far south of the SIE (Figure S19); such info that can highly affect the model inferences. Thus, it is strongly recommended to ensure spatial matching between acoustic detections and environmental conditions, particularly in highly dynamic or heterogeneous environments and for low-frequency calls that can propagate for hundreds of kilometres. Spatial independence: It is important to identify the occurrence of identical calls recorded concurrently at different recording sites. Neglecting this aspect may result in detecting the same call at more than one recording site, leading to a spurious increase in sample size and ambiguities regarding the environmental conditions associated with acoustic presences. Using a minimum received level criterion of 109 dB re 1 μPa to filter daily detections to only keep ABW detections within ~10 km from the recording site (motivated by reasons given in point #3) prevented duplicated detections of the same call at two or more recorders (recorder sites are spaced by >100 km). We strongly recommend maintaining spatial independence between temporally overlapping calls at different recording sites when used in SDMs, particularly for low-frequency calls propagating over long distances (Širović & Hildebrand, 2011). Temporal independence: Temporal independence between consecutive acoustic detections should be considered before used in the models. When the calling animal remains present within the accepted detection range of the acoustic site (as defined by an RL threshold) for long times (e.g., during situations with favourable environmental conditions, availability of plenty of prey or lack of competitors or predators), the same individual may be detected day after day for extended periods of time, which violates the independence assumption of statistical models (Gibb et al., 2019). Temporal dependence is more prominent in low-frequency calls with lower TL than mid- and particularly high-frequency vocalizations. While this bias is somewhat remedied by only including calls produced near the recorders, as implemented here through the applied RL threshold, temporal dependence of callers prevailing in the resulting 10 km radius is overcome by implementing temporal filtering (e.g., we kept a single detection in each 3-day interval). This may increase temporal independence between daily detections unless the calling individual is persistent within a 10 km radius from the site for long time periods. Spatial coverage: Due to the significant operational costs and logistic challenges linked to the deployment and recovery of instruments in the deep sea and remote areas (Rayment et al., 2017), the spatial coverage of PAM is mostly limited to a few, and often opportunistically chosen, recording sites (Brookes et al., 2013; Frasier et al., 2021) by piggy-backing onto moorings addressing other research foci. Ignoring an optimized sampling strategy that samples different strata equally well, however, might introduce biases into the habitat model results. In this study, for example, we only had access to PAM data from five sites near the Greenwich meridian with a focus on the less-explored southern parts of the study area, which may have resulted in low HS suitability in the northern parts of the study area. This is evident in the model evaluation: using the northernmost site (AWI227; Figure 1) to cross-validate ModelPAM led to lower performance, suggesting that this site represents environmental conditions different from other recording sites (Figure S12). Further, the availability of data only from a few sites (as in our case) disallows the efficient use of static predictors in the models, which can be either directly or indirectly important for the species. Other PAM data from the Weddell Sea and off the AP exist but have not been processed yet (see Figure 1 in Thomisch et al., 2016). Integrating PAM data from widely spaced recorders in the SO in future SDMs, for example, the collaborative SOHN network (Southern Ocean Hydrophone Network; van Opzeeland et al., 2014), will be paramount to improving our understanding of the spatiotemporal distribution of ABWs in the SO. 4.4 Combining visual and PAM data in SDMs Combining the complementary visual and acoustic data is promising for monitoring the year-round distribution and trends of ABWs in the SO (Calderan et al., 2020; Miller, Potts, et al., 2019). This approach increases the sample size and maximizes the use of available information, thus covering broad behavioural aspects of the cetacean community and a greater range of environmental conditions that better capture the full realized niche of the species (Dalpaz et al., 2021; Fleming et al., 2018). Integrating both data types for ABWs in a single set of SDMs leverages the relatively broader spatial coverage of visual data and the broader temporal/environmental coverage of acoustic data. Modelcombined predicted high HS at areas highly suitable in both Modelvisual and ModelPAM (Figure 3).
It is important to note, however, that simple data pooling, as implemented here, does not explicitly factor in the differences between the two data types (Fletcher Jr. et al., 2019; Isaac et al., 2020). As a simple alternative to data pooling, independent models, each using one data type, could be run and then combine or average predictions (Fletcher Jr. et al., 2019). Further, one data source could be used as ancillary information for the other; for example, using information from one data source as a prior in a Bayesian framework or using predictions from a model using one data type as a predictor for the second (Fletcher Jr. et al., 2019; Merow et al., 2016; Miller, Pacifici, et al., 2019). However, the sample size and research efforts of visual and acoustic data of baleen whales, particularly from polar areas, are not consistent. While visual surveys span a greater geographic area and range of static environmental predictors, acoustic data has a larger sample size and broader temporal coverage (i.e., is less temporally biased). This can result in PAM data dominating the results of the SDMs (Simmonds et al., 2020; Zipkin et al., 2021). Such dominance of PAM data might be the cause of the noticeable high spatial overlap between Modelcombined and ModelPAM. In future studies, we suggest balancing the number of visual and PAM presences used in the models. This can be done, for example, by randomly sampling the more frequent data source (here PAM) to obtain a balanced number of observations for both data types. This needs to be repeated many times to average stochastic effects. However, this can be challenging for large-scale studies as the data used in the model can be huge. For example, there are a total of ~11.7 million background information used in this study, resulting in high memory demand and long running time of the models even on high-performance computers.
Recent advances in SDMs have focused on integrating different data sources in integrated distribution models (IDMs) (Fletcher Jr. et al., 2019; Pacifici et al., 2017). IDMs acknowledge that data were collected using different protocols, sampling efforts and spatiotemporal biases, thus increasing the models’ predictive accuracy (Fletcher Jr. et al., 2019; Simmonds et al., 2020). IDMs leverage the advantages and minimize the drawbacks of each data source. Further, they were shown to outperform independent models and yield a better understanding of the ecological processes of interest (Isaac et al., 2020; Simmonds et al., 2020; Zipkin et al., 2019). In future applications, we would choose IDMs that combine visual and acoustic data of baleen whales to consider uncertainties in each data source.
ACKNOWLEDGEMENTS We thank Andy Traumüller for helping in downloading environmental predictors and processing ship tracking data and Krissy Anne Reeve for the fruitful discussions on the location and ecological importance of the Weddell Gyre. Special thanks to all data providers for making their observation data publicly available and the nautical officers onboard RV Polarstern who diligently logged their encounters with whales. This work was partially performed on the HPC computer facility of the Alfred Wegener Institute. This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), Grant Number 2817HS004. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), Grant Number 2817HS004.
CONFLICT OF INTEREST STATEMENT Authors have no conflict of interest to declare.
The authors are members of the ocean acoustics group at the Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research. They are interested in exploring the distribution and behaviour of marine mammals and their underwater acoustic environment in the polar oceans using long-term (multi-year) visual observations and passive acoustic recordings. They aim at providing reliable information on year-round distributions of marine mammals in polar oceans to inform conservation, management and science. They are particularly interested in using static and dynamic species distribution models in polar areas using visual and PAM data.
Author contributions: Conceptualization: Ahmed El-Gabbas, Ilse Van Opzeeland, Elke Burkhardt and Olaf Boebel; Data curation, software, formal analysis and visualization: Ahmed El-Gabbas (lead) and Karolin Thomisch (supporting); Funding acquisition, supervision and project administration: Ilse Van Opzeeland, Elke Burkhardt and Olaf Boebel; Methodology: Ahmed El-Gabbas, Karolin Thomisch, Ilse Van Opzeeland and Olaf Boebel. Ahmed El-Gabbas drafted the paper, and all authors have revised and contributed substantially to the interpretation of the results in subsequent drafts.
Atkinson, A., Siegel, V., Pakhomov, E. A., Rothery, P., Loeb, V., Ross, R. M., Quetin, L. B., Schmidt, K., Fretwell, P., Murphy, E. J., Tarling, G. A., & Fleming, A. H. (2008). Oceanic circumpolar habitats of Antarctic krill. Marine Ecology Progress Series, 362, 1–23. https://doi.org/10.3354/meps07498 Attard, C. R., Beheregaray, L. B., & Moller, L. M. (2016). Towards population-level conservation in the critically endangered Antarctic blue whale: The number and distribution of their populations. Scientific Reports, 6, 22291. https://doi.org/10.1038/srep22291 Barlow, D. R., Klinck, H., Ponirakis, D., Branch, T. A., & Torres, L. G. (2023). Environmental conditions and marine heatwaves influence blue whale foraging and reproductive effort. Ecology and Evolution, 13(2), e9770. https://doi.org/10.1002/ece3.9770 Baumgartner, M. F., & Fratantoni, D. M. (2008). Diel periodicity in both sei whale vocalization rates and the vertical migration of their copepod prey observed from ocean gliders. Limnology and Oceanography, 53(5part2), 2197–2209. https://doi.org/10.4319/lo.2008.53.5_part_2.2197 Bombosch, A., Zitterbart, D. P., Van Opzeeland, I., Frickenhaus, S., Burkhardt, E., Wisz, M. S., & Boebel, O. (2014). Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part I: Oceanographic Research Papers, 91, 101–114. https://doi.org/10.1016/j.dsr.2014.05.017 Branch, T. A. (2007). Abundance of Antarctic blue whales south of 60° S from three complete circumpolar sets of surveys. Journal of Cetacean Research and Management, 9(3), 253–262. Branch, T. A. (2008). Current status of Antarctic blue whales based on Bayesian modeling. Report (SC/60/SH7) to the Scientific Committee of the International Whaling Commission. Branch, T. A., Abubaker, E. M. N., Mkango, S., & Butterworth, D. S. (2007). Separating southern blue whale subspecies based on length frequencies of sexually mature females. Marine Mammal Science, 23(4), 803–833. https://doi.org/10.1111/j.1748-7692.2007.00137.x Branch, T. A., Matsuoka, K., & Miyashita, T. (2004). Evidence for increases in Antarctic blue whales based on Bayesian modelling. Marine Mammal Science, 20(4), 726–754. https://doi.org/10.1111/j.1748-7692.2004.tb01190.x Branch, T. A., Stafford, K. M., Palacios, D. M., Allison, C., Bannister, J. L., Burton, C. L. K., Cabrera, E., Carlson, C. A., Galletti Vernazzani, B., Gill, P. C., Hucke-Gaete, R., Jenner, K. C., Jenner, M. N. M., Matsuoka, K., Mikhalev, Y. A., Miyashita, T., Morrice, M. G., Nishiwaki, S., Sturrock, V. J., … Warneke, R. M. (2007). Past and present distribution, densities and movements of blue whales Balaenoptera musculus in the southern hemisphere and northern Indian Ocean. Mammal Review, 37(2), 116–175. https://doi.org/10.1111/j.1365-2907.2007.00106.x Brookes, K. L., Bailey, H., & Thompson, P. M. (2013). Predictions from harbor porpoise habitat association models are confirmed by long-term passive acoustic monitoring. The Journal of the Acoustical Society of America, 134(3), 2523–2533. https://doi.org/10.1121/1.4816577 Burham, R. E., Palm, R. S., Duffus, D. A., Mouy, X., & Riera, A. (2016). The combined use of visual and acoustic data collection techniques for winter killer whale (Orcinus orca) observations. Global Ecology and Conservation, 8, 24–30. https://doi.org/10.1016/j.gecco.2016.08.001 Calderan, S. V., Black, A., Branch, T. A., Collins, M. A., Kelly, N., Leaper, R., Lurcock, S., Miller, B. S., Moore, M., Olson, P. A., Širović, A., Wood, A. G., & Jackson, J. A. (2020). South Georgia blue whales five decades after the end of whaling. Endangered Species Research, 43, 359–373. https://doi.org/10.3354/esr01077 Calderan, S., Miller, B., Collins, K., Ensor, P., Double, M., Leaper, R., & Barlow, J. (2014). Low-frequency vocalizations of sei whales (Balaenoptera borealis) in the Southern Ocean. The Journal of the Acoustical Society of America, 136(6), EL418. https://doi.org/10.1121/1.4902422 Clapham, P. J., & Baker, C. S. (2009). Whaling, modern. In B. Würsig, J. G. M. Thewissen, & K. M. Kovacs (Eds.), Encyclopedia of marine mammals (pp. 1239–1243). Academic Press. Cooke, J. G. (2018). Balaenoptera musculus ssp. intermedia. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T41713A50226962.en Cuzin-Roudy, J., Irisson, J.-O., Penot, F., Kawaguchi, S., & Vallet, C. (2014). Southern Ocean euphausiids. In C. De Broyer, P. Koubbi, H. J. Griffiths, B. Raymond, C. d. Udekem d’Acoz, A. P. Putte, B. Danis, B. David, S. Grant, J. Gutt, C. Held, G. Hosie, F. Huettmann, A. Post, & Y. Ropert-Coudert (Eds.), Biogeographic atlas of the Southern Ocean (pp. 309–320). Scientific Committee on Antarctic Research. Dalpaz, L., Paro, A. D., Daura-Jorge, F. G., Rossi-Santos, M., Norris, T. F., Ingram, S. N., & Wedekin, L. L. (2021). Better together: Analysis of integrated acoustic and visual methods when surveying a cetacean community. Marine Ecology Progress Series, 678, 197–209. https://doi.org/10.3354/meps13898 Darling, J. D. (2015). Low frequency, ca. 40 Hz, pulse trains recorded in the humpback whale assembly in Hawaii. The Journal of the Acoustical Society of America, 138(5), EL452–EL458. https://doi.org/10.1121/1.4935070 Dominello, T., & Širović, A. (2016). Seasonality of Antarctic minke whale (Balaenoptera bonaerensis) calls off the western Antarctic peninsula. Marine Mammal Science, 32(3), 826–838. https://doi.org/10.1111/mms.12302 Double, M. C., Miller, B. S., Leaper, R., Olson, P., Cox, M. J., Miller, E., Calderan, S., Collins, K., Donnelly, D., Ensor, P., Goetz, K., Schmitt, N., Andrews-Goff, V., Bell, E., … O’Driscoll, R. (2015). Cruise report on blue whale research from the NZ/Aus Antarctic ecosystems voyage 2015 of the Southern Ocean research partnership. Paper SC/66a/SH/7 presented to the IWC Scientific Committee. https://iwc.int/ El-Gabbas, A., & Dormann, C. F. (2018). Improved species-occurrence predictions in data-poor regions: Using large-scale data and bias correction with down-weighted Poisson regression and maxent. Ecography, 41(7), 1161–1172. https://doi.org/10.1111/ecog.03149 El-Gabbas, A., Thomisch, K., Van opzeeland, I., Burkhardt, E., & Boebel, O. (2023). Dynamic habitat suitability of Antarctic blue whales (Balaenoptera musculus intermedia) in the Weddell Sea using visual and PAM data. https://doi.org/10.6084/m9.figshare.21944957 El-Gabbas, A., Van Opzeeland, I., Burkhardt, E., & Boebel, O. (2021a). Dynamic species distribution models in the marine realm: Predicting year-round habitat suitability of baleen whales in the Southern Ocean. Frontiers in Marine Science, 8, Article 802276. https://doi.org/10.3389/fmars.2021.802276 El-Gabbas, A., Van Opzeeland, I., Burkhardt, E., & Boebel, O. (2021b). Static species distribution models in the marine realm: The case of baleen whales in the Southern Ocean. Diversity and Distributions, 27(8), 1536–1552. https://doi.org/10.1111/ddi.13300 Fleming, A. H., Yack, T., Redfern, J. V., Becker, E. A., Moore, T. J., & Barlow, J. (2018). Combining acoustic and visual detections in habitat models of Dall’s porpoise. Ecological Modelling, 384, 198–208. https://doi.org/10.1016/j.ecolmodel.2018.06.014 Fletcher, R. J., Jr., Hefley, T. J., Robertson, E. P., Zuckerberg, B., McCleery, R. A., & Dorazio, R. M. (2019). A practical guide for combining data to model species distributions. Ecology, 100(6), e02710. https://doi.org/10.1002/ecy.2710 Frasier, K. E., Garrison, L. P., Soldevilla, M. S., Wiggins, S. M., & Hildebrand, J. A. (2021). Cetacean distribution models based on visual and passive acoustic data. Scientific Reports, 11(1), 8240. https://doi.org/10.1038/s41598-021-87577-1 Gavrilov, A. N., McCauley, R. D., Salgado-Kent, C., Tripovich, J., & Burton, C. (2011). Vocal characteristics of pygmy blue whales and their change over time. The Journal of the Acoustical Society of America, 130(6), 3651–3660. https://doi.org/10.1121/1.3651817 Gibb, R., Browning, E., Glover-Kapfer, P., & Jones, K. E. (2019). Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods in Ecology and Evolution, 10(2), 169–185. https://doi.org/10.1111/2041-210x.13101 Guisan, A., Thuiller, W., & Zimmermann, N. E. (2017). Habitat suitability and distribution models, with applications in R. Cambridge University Press. Harmer, S. F. (1931). Southern whaling. Proceedings of the Linnean Society of London, 142(1), 85–163. https://doi.org/10.1111/j.1095-8312.1931.tb01464.x Hinton, M. A. C. (1915). Report on the papers left by the late Major Barrett-Hamilton, relating to the whales of South Georgia. In Inter-departmental committee on whaling and the protection of whales (appendix VII). Colonial office miscellaneous number 298 (pp. 269–293). Crown Agents for the Colonies. Hofmann, E. E., Klinck, J. M., Locarnini, R. A., Fach, B., & Murphy, E. (2004). Krill transport in the Scotia Sea and environs. Antarctic Science, 10(4), 406–415. https://doi.org/10.1017/s0954102098000492 Horwood, J. W. (1986). The distribution of the southern blue whale in relation to recent estimates and abundance. Scientific Reports of the Whales Research Institute, 37, 155–165. Ichihara, T. (1966). 6. The pygmy blue whale, Balaenoptera musculus brevicauda, a new subspecies from the Antarctic. In K. S. Norris (Ed.), Whales, dolphins, and porpoises (pp. 79–113). Isaac, N. J. B., Jarzyna, M. A., Keil, P., Dambly, L. I., Boersch-Supan, P. H., Browning, E., Freeman, S. N., Golding, N., Guillera-Arroita, G., Henrys, P. A., Jarvis, S., Lahoz-Monfort, J., Pagel, J., Pescott, O. L., Schmucki, R., Simmonds, E. G., & O’Hara, R. B. (2020). Data integration for large-scale models of species distributions. Trends in Ecology & Evolution, 35(1), 56–67. https://doi.org/10.1016/j.tree.2019.08.006 JPL MUR MEaSUREs Project. (2015). GHRSST level 4 MUR global Foundation Sea surface temperature analysis (v4.1) [PO.DAAC, CA, USA]. https://doi.org/10.5067/GHGMR-4FJ04 Kasamatsu, F., Hembree, D., Joyce, G., Tsunoda, L., Rowlett, R., & Nakano, T. (1988). Distribution of cetacean sightings in the Antarctic: Results obtained from the IWC/IDCR minke whale assessment cruises, 1978/79 to 1983/84. Paper SC/39/OI0 presented to the IWC Scientific Committee. https://iwc.int/ Kasamatsu, F., Matsuoka, K., & Hakamada, T. (2000). Interspecific relationships in density among the whale community in the Antarctic. Polar Biology, 23(7), 466–473. https://doi.org/10.1007/s003009900107 Kaschner, K., Quick, N. J., Jewell, R., Williams, R., & Harris, C. M. (2012). Global coverage of cetacean line-transect surveys: Status quo, data gaps and future challenges. PLoS One, 7(9), e44075. https://doi.org/10.1371/journal.pone.0044075 Kemp, S., & Bennett, A. G. (1932). On the distribution and movements of whales on the South Georgia and south Shetland whaling grounds. Discovery Reports, 7, 167–190. Klinck, H., Mellinger, D. K., Klinck, K., Hager, J., Kindermann, L., & Boebel, O. (2010). Long-range underwater vocalizations of the crabeater seal (Lobodon carcinophaga). The Journal of the Acoustical Society of America, 128(1), 474–479. https://doi.org/10.1121/1.3442362 Laws, R. M. (1985). The ecology of the Southern Ocean. American Scientist, 73(1), 26–40. Leaper, R., & Miller, C. (2011). Management of Antarctic baleen whales amid past exploitation, current threats and complex marine ecosystems. Antarctic Science, 23(6), 503–529. https://doi.org/10.1017/s0954102011000708 Leroy, E. C., Samaran, F., Bonnel, J., & Royer, J. Y. (2016). Seasonal and diel vocalization patterns of Antarctic blue whale (Balaenoptera musculus intermedia) in the southern Indian Ocean: A multi-year and multi-site study. PLoS One, 11(11), e0163587. https://doi.org/10.1371/journal.pone.0163587 Letsheleha, I. S., Shabangu, F. W., Farrell, D., Andrew, R. K., la Grange, P. L., & Findlay, K. P. (2022). Year-round acoustic monitoring of Antarctic blue and fin whales in relation to environmental conditions off the west coast of South Africa. Marine Biology, 169(3), Article 41. https://doi.org/10.1007/s00227-022-04026-x Lewis, L. A., Calambokidis, J., Stimpert, A. K., Fahlbusch, J., Friedlaender, A. S., McKenna, M. F., Mesnick, S. L., Oleson, E. M., Southall, B. L., Szesciorka, A. R., & Sirovic, A. (2018). Context-dependent variability in blue whale acoustic behaviour. Royal Society Open Science, 5(8), 180241. https://doi.org/10.1098/rsos.180241 Liu, C., White, M., Newell, G., & Pearson, R. (2013). Selecting thresholds for the prediction of species occurrence with presence-only data. Journal of Biogeography, 40(4), 778–789. https://doi.org/10.1111/jbi.12058 Ljungblad, D. K., Clark, C. W., & Shimada, H. (1998). A comparison of sounds attributed to pygmy blue whales (Balaenoptera musculus brevicauda) recorded south of the Madagascar plateau and those attributed to ‘true’ blue whales (Balaenoptera musculus) recorded off Antarctica. Reports of the IWC Scientific Committee, 48, 439–442. Lobo, J. M., Jiménez-Valverde, A., & Real, R. (2008). AUC: A misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography, 17(2), 145–151. https://doi.org/10.1111/j.1466-8238.2007.00358.x Matsuoka, K., Watanabe, T., Ichii, T., Shimada, H., & Nishiwaki, S. (2003). Large whale distributions (south of 60° S, 35° E-130° E) in relation to the southern boundary of the Antarctic circumpolar current. In A. H. L. Huiskes, W. W. C. Gieskes, J. Rozema, R. M. L. Schorno, S. M. Vies, & W. J. Wolff (Eds.), Antarctic biology in a global context. Backhuys Publishers. Mellinger, D. K., & Clark, C. W. (2000). Recognizing transient low-frequency whale sounds by spectrogram correlation. The Journal of the Acoustical Society of America, 107(6), 3518–3529. https://doi.org/10.1121/1.429434 Mellinger, D. K., Stafford, K. M., Moore, S. E., Dziak, R. P., & Matsumoto, H. (2007). An overview of fixed passive acoustic observation methods for cetaceans. Oceanography, 20(4), 36–45. https://doi.org/10.5670/oceanog.2007.03 Merow, C., Allen, J. M., Aiello-Lammens, M., & Silander, J. A. (2016). Improving niche and range estimates with maxent and point process models by integrating spatially explicit information. Global Ecology and Biogeography, 25(8), 1022–1036. https://doi.org/10.1111/geb.12453 Miller, B. S., Barlow, J., Calderan, S., Collins, K., Leaper, R., Olson, P., Ensor, P., Peel, D., Donnelly, D. M., Andrews-Goff, V., Olavarría, C., Owen, K., Rekdahl, M., Schmitt, N., Wadley, V., Gedamke, J., Gales, N., & Double, M. C. (2015). Validating the reliability of passive acoustic localisation: A novel method for encountering rare and remote Antarctic blue whales. Endangered Species Research, 26(3), 257–269. https://doi.org/10.3354/esr00642 Miller, B. S., Calderan, S., Leaper, R., Miller, E. J., Sirovic, A., Stafford, K. M., Bell, E., & Double, M. C. (2021). Source level of Antarctic blue and fin whale sounds recorded on Sonobuoys deployed in the Deep-Ocean off Antarctica. Frontiers in Marine Science, 8, Article 792651. https://doi.org/10.3389/fmars.2021.792651 Miller, B. S., Calderan, S., Miller, E. J., Širović, A., Stafford, K. M., Bell, E., & Double, M. C. (2019). A passive acoustic survey for marine mammals conducted during the 2019 Antarctic voyage on Euphausiids and Nutrient Recycling in Cetacean Hotspots (ENRICH). Proceedings of ACOUSTICS 2019. https://par.nsf.gov/biblio/10134677 Miller, B. S., Leaper, R., Calderan, S., & Gedamke, J. (2014). Red shift, blue shift: Investigating doppler shifts, blubber thickness, and migration as explanations of seasonal variation in the tonality of Antarctic blue whale song. PLoS One, 9(9), e107740. https://doi.org/10.1371/journal.pone.0107740 Miller, B. S., Madhusudhana, S., Aulich, M. G., Kelly, N., Lecours, V., & Risch, D. (2023). Deep learning algorithm outperforms experienced human observer at detection of blue whale D-calls: A double-observer analysis. Remote Sensing in Ecology and Conservation, 9(1), 104–116. https://doi.org/10.1002/rse2.297 Miller, D. A. W., Pacifici, K., Sanderlin, J. S., & Reich, B. J. (2019). The recent past and promising future for data integration methods to estimate species’ distributions. Methods in Ecology and Evolution, 10(1), 22–37. https://doi.org/10.1111/2041-210x.13110 Miller, E. J., Potts, J. M., Cox, M. J., Miller, B. S., Calderan, S., Leaper, R., Olson, P. A., O’Driscoll, R. L., & Double, M. C. (2019). The characteristics of krill swarms in relation to aggregating Antarctic blue whales. Scientific Reports, 9(1), 16487. https://doi.org/10.1038/s41598-019-52792-4 Moore, S. E., Stafford, K. M., Melling, H., Berchok, C., Wiig, Ø., Kovacs, K. M., Lydersen, C., & Richter-Menge, J. (2011). Comparing marine mammal acoustic habitats in Atlantic and Pacific sectors of the high Arctic: Year-long records from Fram Strait and the Chukchi plateau. Polar Biology, 35(3), 475–480. https://doi.org/10.1007/s00300-011-1086-y Oleson, E. M., Calambokidis, J., Burgess, W. C., McDonald, M. A., LeDuc, C. A., & Hildebrand, J. A. (2007). Behavioral context of call production by eastern North Pacific blue whales. Marine Ecology Progress Series, 330, 269–284. https://doi.org/10.3354/meps330269 Oleson, E. M., Wiggins, S. M., & Hildebrand, J. A. (2007). Temporal separation of blue whale call types on a southern California feeding ground. Animal Behaviour, 74(4), 881–894. https://doi.org/10.1016/j.anbehav.2007.01.022 Orsi, A. H., Whitworth, T., & Nowlin, W. D. (1995). On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Research Part I: Oceanographic Research Papers, 42(5), 641–673. https://doi.org/10.1016/0967-0637(95)00021-W Paarman, S., Vermeulen, E., Seyboth, E., Thornton, M., & Findlay, K. (2021). Abundance and distribution of Antarctic blue whales Balaenoptera musculus intermedia off the queen Maud land coast of Antarctica. African Journal of Marine Science, 43(1), 53–59. https://doi.org/10.2989/1814232x.2020.1864471 Pacifici, K., Reich, B. J., Miller, D. A., Gardner, B., Stauffer, G., Singh, S., McKerrow, A., & Collazo, J. A. (2017). Integrating multiple data sources in species distribution modeling: A framework for data fusion. Ecology, 98(3), 840–850. https://doi.org/10.1002/ecy.1710 Phillips, S. J., Anderson, R. P., Dudik, M., Schapire, R. E., & Blair, M. E. (2017). Opening the black box: An open-source release of maxent. Ecography, 40(7), 887–893. https://doi.org/10.1111/ecog.03049 Rankin, S., Ljungblad, D., Clark, C., & Kato, H. (2005). Vocalisations of Antarctic blue whales, Balaenoptera musculus intermedia, recorded during the 2001/2002 and 2002/2003 IWC/SOWER circumpolar cruises, area V, Antarctica. Journal of Cetacean Research and Management, 7(1), 13–20. Rayment, W., Webster, T., Brough, T., Jowett, T., & Dawson, S. (2017). Seen or heard? A comparison of visual and acoustic autonomous monitoring methods for investigating temporal variation in occurrence of southern right whales. Marine Biology, 165(1), Article 12. https://doi.org/10.1007/s00227-017-3264-0 Renner, I. W., Elith, J., Baddeley, A., Fithian, W., Hastie, T., Phillips, S. J., Popovic, G., & O’Hara, R. B. (2015). Point process models for presence-only analysis. Methods in Ecology and Evolution, 6(4), 366–379. https://doi.org/10.1111/2041-210x.12352 Rettig, S., Boebel, O., Menze, S., Kindermann, L., Thomisch, K., & van Opzeeland, I. (2013). Local to basin scale arrays for passive acoustic monitoring in the Atlantic sector of the Southern Ocean. Paper presented at the 1st International Conference and Exhibition on Underwater Acoustic. https://epic.awi.de/id/eprint/33846/ Risting, S. (1928). Whales and whale foetuses: Statistics of catch and measurements collected from the Norwegian Whalers’ Association 1922–1925. Rapports et Procès-Verbaux des Réunions, 50, 51–122. Robinson, L. M., Elith, J., Hobday, A. J., Pearson, R. G., Kendall, B. E., Possingham, H. P., & Richardson, A. J. (2011). Pushing the limits in marine species distribution modelling: Lessons from the land present challenges and opportunities. Global Ecology and Biogeography, 20(6), 789–802. https://doi.org/10.1111/j.1466-8238.2010.00636.x Ruud, J. T. (1956). The blue whale. Scientific American, 195(6), 46–50. https://doi.org/10.1038/scientificamerican1256-46 Samaran, F., Adam, O., & Guinet, C. (2010). Discovery of a mid-latitude sympatric area for two southern hemisphere blue whale subspecies. Endangered Species Research, 12(2), 157–165. https://doi.org/10.3354/esr00302 Scheidat, M., Friedlaender, A., Kock, K. H., Lehnert, L., Boebel, O., Roberts, J., & Williams, R. (2011). Cetacean surveys in the Southern Ocean using icebreaker-supported helicopters. Polar Biology, 34(10), 1513–1522. https://doi.org/10.1007/s00300-011-1010-5 Shabangu, F. W., Andrew, R. K., Yemane, D., & Findlay, K. P. (2020). Acoustic seasonality, behaviour and detection ranges of Antarctic blue and fin whales under different sea ice conditions off Antarctica. Endangered Species Research, 43, 21–37. https://doi.org/10.3354/esr01050 Shabangu, F. W., Yemane, D., Stafford, K. M., Ensor, P., & Findlay, K. P. (2017). Modelling the effects of environmental conditions on the acoustic occurrence and behaviour of Antarctic blue whales. PLoS One, 12(2), e0172705. https://doi.org/10.1371/journal.pone.0172705 Siegel, V. (2005). Distribution and population dynamics of Euphausia superba: Summary of recent findings. Polar Biology, 29(1), 1–22. https://doi.org/10.1007/s00300-005-0058-5 Simard, Y., Roy, N., & Gervaise, C. (2008). Passive acoustic detection and localization of whales: Effects of shipping noise in Saguenay-St. Lawrence Marine Park. The Journal of the Acoustical Society of America, 123(6), 4109–4117. https://doi.org/10.1121/1.2912453 Simmonds, E. G., Jarvis, S. G., Henrys, P. A., Isaac, N. J. B., & O’Hara, R. B. (2020). Is more data always better? A simulation study of benefits and limitations of integrated distribution models. Ecography, 43(10), 1413–1422. https://doi.org/10.1111/ecog.05146 Slijper, E. J. (1962). Whales/translated by a.J. Pomerans. Basic Books. Smith, J. N., Kelly, N., & Renner, I. W. (2020). Validation of presence-only models for conservation planning and the application to whales in a multiple-use marine park. Ecological Applications, 31, e02214. https://doi.org/10.1002/eap.2214 Sofaer, H. R., Hoeting, J. A., & Jarnevich, C. S. (2019). The area under the precision-recall curve as a performance metric for rare binary events. Methods in Ecology and Evolution, 10(4), 565–577. https://doi.org/10.1111/2041-210x.13140 Soldevilla, M. S., Wiggins, S. M., & Hildebrand, J. A. (2010). Spatial and temporal patterns of Risso’s dolphin echolocation in the Southern California bight. The Journal of the Acoustical Society of America, 127(1), 124–132. https://doi.org/10.1121/1.3257586 Spreen, G., Kaleschke, L., & Heygster, G. (2008). Sea ice remote sensing using AMSR-E 89-GHz channels. Journal of Geophysical Research-Oceans, 113(C2), Article C02S03. https://doi.org/10.1029/2005jc003384 Stanton, J. C., Pearson, R. G., Horning, N., Ersts, P., & Reşit Akçakaya, H. (2012). Combining static and dynamic variables in species distribution models under climate change. Methods in Ecology and Evolution, 3(2), 349–357. https://doi.org/10.1111/j.2041-210X.2011.00157.x Thomas, P. G., & Green, K. (1988). Distribution of Euphausia crystallorophias within Prydz Bay and its importance to the inshore marine ecosystem. Polar Biology, 8(5), 327–331. https://doi.org/10.1007/bf00442023 Thomisch, K. (2017). Distribution patterns and migratory behavior of Antarctic blue whales. Berichte Zur Polar – Und Meeresforschung – Reports on Polar and Marine Research. Thomisch, K., Boebel, O., Clark, C. W., Hagen, W., Spiesecke, S., Zitterbart, D. P., & Van Opzeeland, I. (2016). Spatio-temporal patterns in acoustic presence and distribution of Antarctic blue whales Balaenoptera musculus intermedia in the Weddell Sea. Endangered Species Research, 30, 239–253. https://doi.org/10.3354/esr00739 Thomisch, K., El-Gabbas, A., Spiesecke, S., & Boebel, O. (2023). Daily acoustic presence of Antarctic blue whales (Balaenoptera musculus intermedia) within a 10 km radius of recording sites in the Weddell Sea, based on passive acoustic monitoring data from 2008 to 2013. Pangaea. https://doi.org/10.1594/PANGAEA.959969 Thompson, P. O., Findley, L. T., & Vidal, O. (1992). 20-Hz pulses and other vocalizations of fin whales, Balaenoptera physalus, in the Gulf of California, Mexico. The Journal of the Acoustical Society of America, 92(6), 3051–3057. https://doi.org/10.1121/1.404201 Torterotot, M., Samaran, F., Stafford, K. M., & Royer, J.-Y. (2020). Distribution of blue whale populations in the southern Indian Ocean based on a decade of acoustic monitoring. Deep Sea Research Part II: Topical Studies in Oceanography, 179, 104874. https://doi.org/10.1016/j.dsr2.2020.104874 Tynan, C. T. (1998). Ecological importance of the southern boundary of the Antarctic circumpolar current. Nature, 392(6677), 708–710. https://doi.org/10.1038/33675 Van Opzeeland, I., Van Parijs, S., Kindermann, L., Burkhardt, E., & Boebel, O. (2013). Calling in the cold: Pervasive acoustic presence of humpback whales (Megaptera novaeangliae) in Antarctic coastal waters. PLoS One, 8(9), e73007. https://doi.org/10.1371/journal.pone.0073007 Warren, D. L., Glor, R. E., & Turelli, M. (2008). Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution, 62(11), 2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x Wiedenmann, J., Cresswell, K. A., Goldbogen, J., Potvin, J., & Mangel, M. (2011). Exploring the effects of reductions in krill biomass in the Southern Ocean on blue whales using a state-dependent foraging model. Ecological Modelling, 222(18), 3366–3379. https://doi.org/10.1016/j.ecolmodel.2011.07.013 Williams, R., Hedley, S. L., & Hammond, P. S. (2006). Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecology and Society, 11(1), 1. Wyles, H. M. E., Boehme, L., Russell, D. J. F., & Carter, M. I. D. (2022). A novel approach to using seabed geomorphology as a predictor of habitat use in highly mobile marine predators: Implications for ecology and conservation. Frontiers in Marine Science, 9, Article 818635. https://doi.org/10.3389/fmars.2022.818635 Zipkin, E. F., Inouye, B. D., & Beissinger, S. R. (2019). Innovations in data integration for modeling populations. Ecology, 100(6), e02713. https://doi.org/10.1002/ecy.2713 Zipkin, E. F., Zylstra, E. R., Wright, A. D., Saunders, S. P., Finley, A. O., Dietze, M. C., Itter, M. S., & Tingley, M. W. (2021). Addressing data integration challenges to link ecological processes across scales. Frontiers in Ecology and the Environment, 19(1), 30–38. https://doi.org/10.1002/fee.2290 van Opzeeland, I., Samaran, F., Stafford, K. M., Findlay, K., Gedamke, J., Harris, D., & Miller, B. S. (2014). Towards collective circum-Antarctic passive acoustic monitoring: The Southern Ocean hydrophone network (SOHN). Polarforschung, 83(2), 47–61. Širović, A., & Hildebrand, J. A. (2011). Using passive acoustics to model blue whale habitat off the Western Antarctic peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 58(13–16), 1719–1728. https://doi.org/10.1016/j.dsr2.2010.08.019 Širović, A., Hildebrand, J. A., & Wiggins, S. M. (2007). Blue and fin whale call source levels and propagation range in the Southern Ocean. The Journal of the Acoustical Society of America, 122(2), 1208–1215. https://doi.org/10.1121/1.2749452 Širović, A., Hildebrand, J. A., Wiggins, S. M., & Thiele, D. (2009). Blue and fin whale acoustic presence around Antarctica during 2003 and 2004. Marine Mammal Science, 25(1), 125–136. https://doi.org/10.1111/j.1748-7692.2008.00239.x Širović, A., Hildebrand, J. A., Wiggins, S. M., McDonald, M. A., Moore, S. E., & Thiele, D. (2004). Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 51(17–19), 2327–2344. https://doi.org/10.1016/j.dsr2.2004.08.005
Supplementary materials for:
Figure S1: Temporal distribution of Antarctic blue whale detections.
Plots show the number of daily detections per calendar day (A: visual sightings (2003-2019) from the Weddell Sea; B: acoustic detections at the five stations; C: acoustic detections at each of the five stations). Most visual data were observed from December to mid-February (with only a single sighting in late February, two in March, one in April, and two in November).
Figure S2: Year-round distribution of Antarctic blue whales’ acoustic detections (blue) and non-detections (red), grouped by station ID and year. Grey x symbols represent days with no acoustic data (e.g., due to device failure).
Figure S3: A comparison between predicted habitat suitability of ModelPAM and Modelcombined using the unfiltered (daily) or temporally-filtered (using a single acoustic presence per 3-days interval) acoustic presences. Each map shows predicted habitat suitability on the 15th of each month in 2013. a) sea ice concentration b) distance to the sea ice edge c) lagged sea ice d) sea surface temperature e) sea surface height f) current speed
Figure S4: Predictors’ values at visual and PAM dataset. In each plot, the bottom five rows show the predictor’s value at each acoustic station, with days with acoustic presences marked with blue bars and days with no acoustic detections in light grey bars. In the top row, red bars represent values at visual sightings, while dark grey shows the distribution of the values of the respective predictor in the background information. See Figure 1 and Table 1 for more information on the PAM data.
Figure S5: Spatial blocks used to cross-validate the visual data models (Modelvisual). The study area is divided into larger blocks, each consisting of 25×25 cells (250×250 km). Blocks were distributed into four cross-validation folds (different colors). Antarctic blue whales’ sightings (2003-2019) are shown in black dots. a) Visual-data models Only dynamic predictors (this study) b) Visual-data models Circumpolar models, with both static and dynamic predictors (El-Gabbas et al. 2021a) c) PAM-data models d) Combined-data models
Figure S6: Examples of Antarctic blue whales’ predicted habitat suitability on the 15th day of each month in 2013. Black lines represent the estimated location of the sea ice edge in the respective day.
Figure S7: Overlap between monthly suitable habitats of the Antarctic blue whales in the Weddell Sea using the three model types. Daily suitable habitats (2003-2019) were identified using the model-specific threshold that maximizes the sum of sensitivity and specificity. Cells were identified as suitable on a particular day if they were predicted to be suitable in more than half of the cross-validated models. Daily suitable habitats were then summed in the respective month, and cells were identified as suitable if these cells were predicted to be suitable in ≥25% of days in the respective month. Cells representing monthly suitable habitats were converted to polygons and smoothed to represent the overall pattern of suitable habitats in the respective month. Note that small polygons (with areas less than 1000 km2) were removed from the plots to make the comparisons easy. a) Visual-data models Only dynamic predictors (this study) b) Visual-data models Circumpolar models, with both static and dynamic predictors (El-Gabbas et al. 2021a) c) PAM-data models d) Combined-data models
Figure S8: Biweekly summary of predicted habitat suitability of Antarctic blue whales of the four model types implemented. Each map shows the 90th quantile of daily predicted habitat suitability in the respective two-weeks period and model type from 2003 to 2019. Black circles indicate the location of PAM recorders. These circles are filled with red color if there are any acoustic presences in the respective two-weeks period. Red triangles show visual sightings in the respective two-weeks period. Monthly summary maps are shown in Figure 3. Examples of daily habitat suitability maps on the 15th day of each month in 2013 are shown in Figure S6.
Figure S9: Single variable response curves for Antarctic blue whales’ models. To create these plots, an additional set of models was run only using one predictor in turn. Lines and shaded areas represent the mean and standard deviation of response curves on cross-validation, with different colors for each model type. In each plot, the top rugs show the spatiotemporally matched conditions at species detections (visual sighting in blue, PAM data in red), while the bottom green rug shows values at a sample of background locations from the Weddell Sea (10% of background information used to run the models). Environmental values at PAM detections, grouped by the location of acoustic stations, are shown in Figure S4. See Figure 5 for marginal response curves and Figure S10 for summary values of predicted habitat suitability in the environmental space of pairwise predictors.
Figure S10: Predicted habitat suitability of Antarctic blue whales in pairwise environmental space of the six dynamic predictors used in the models. Columns represent the three models implemented (Modelvisual, ModelPAM, and Modelcombined, respectively). Each plot shows predicted values at year-round environmental combinations (11.7 million background locations spatiotemporally sampled from the Weddell Sea). Predictions at each combination of two predictors (represented as 100×100 pixels) were summarized to represent the 90th quantile of predicted habitat suitability per combination. Pixel colors range from blue to red (low to high habitat suitability), with white pixels for non-existent combinations in the background information. These pairwise comparisons allow all other predictors to vary together to represent pairwise habitat suitability at all other possible combinations in the study area. Spatiotemporally-matched environmental conditions at respective species detections are shown as black points.
Figure S11: Spatial overlap (Schoener’s D metric (Warren et al. 2008) and Pearson’s correlation coefficient) between daily mean predicted habitat suitability of the three model types. Blue dots represent daily overlap values between Modelvisual & ModelPAM; red for Modelvisual & Modelcombined; and grey for ModelPAM & Modelcombined. Black lines show the mean overlap value of the respective model combination for each calendar day. Schoener’s D metric ranges from zero for no overlap to one for complete overlap. For similar results using Warren’s I metric, see Figure 6. A. Visual-data models (cross-validation evaluation) B. PAM-data models (cross-validation evaluation) C. combined-data models (cross-validation evaluation)
Figure S12: Histograms for the calculated 1,000 raw values of cross-validated evaluation: testing AUC (left plots) and testing TSS (right plots) for (a) Modelvisual; (b) ModelPAM; and (c) Modelcombined. The mean and standard deviation of the metric in the respective model and cross-validation fold is shown in the top right of each plot; the mean value is shown as a vertical dashed line. The overall summary of the evaluation metrics is shown in Table 2. For more information on the spatial blocks used to cross-validate Modelvisual, see Figure S5. The location of the PAM stations used to cross-validate ModelPAM is shown in Figure 1. A. Visual-data models, evaluated with PAM data B. PAM-data models, evaluated with visual data
Figure S13: Histograms for the calculated 1,000 raw values of external evaluation: testing AUC (left plots) and testing TSS (right plots) for (a) cross-validated visual-data models (Modelvisual), evaluated with independent PAM data; (b) cross-validated PAM-data models (ModelPAM), evaluated with independent visual sightings data. The mean and standard deviation of the metric in the respective model and cross-validation fold is shown in the top right of each plot; the mean value is shown as a vertical dashed line. The overall summary of the evaluation metrics is shown in Table 2. For more information on the spatial blocks used to cross-validate Modelvisual, see Figure S5. The location of the PAM stations used to cross-validate ModelPAM is shown in Figure 1.
Figure S14: Number of days (log10 scale) each cell was identified as polynya (2003-2019). Daily polynyas (areas with SIC ≤ 15% south of the SIE) were determined by two rules: ≥ 20 connected cells (>2,000 km2) and persistent for at least five consecutive days (two days before and two days after each selected day); see El-Gabbas et al. 2021a for more details. Daily polynyas were converted into a daily binary map (polynya/non-polynya); then, for each cell and month combination, the number of days intersected with polynyas was identified. For the location of the SIE in the respective month, see Figure S15.
Figure S15: The estimated location of daily sea ice edge from 2003 to 2019 in each month (a) and irrespective of the month (b). The area south of the Weddell Basin is permanently covered with high sea ice (sea ice concentration >15% during the whole period), except near the Brunt and Larsen Ice Shelves. This area was predicted unsuitable for the Antarctic blue whales in all models, except sporadically off the Brunt Ice Shelf.
Figure S16: Spatial overlap between daily mean predicted habitat suitability of the Antarctic blue whales in the Weddell Sea visual sightings (D: Schoener’s D metric; I: Warren’s I (Warren et al. 2008) and R: Pearson’s correlation coefficient). This plot dictates the overlap between 1) models run in the Weddell Sea using only dynamic predictors (this study, Modelvisual) and 2) models that used circum-Antarctic visual sightings along with both static and dynamic predictors (El-Gabbas et al. 2021a), cropped to the Weddell Sea study area. Dots represent daily overlap values between models, while lines show the mean overlap value for each calendar day. Values of D and I range from zero for no overlap to one for complete overlap.
Figure S17: Number of background locations used to train the models (log10 scale) at environmental conditions beyond values at acoustic presences: (A) SST > 1.6°C; (B) SSH > −1.1m; and (C) Current speed > 0.1 ms-1. These plots explain, for example, why models predicted low HS values north of the sbACC. These areas have environmental conditions beyond conditions at mooring stations.
Figure S18: The left map shows mean predicted ABWs’ habitat suitability on 27th April 2017. The right plot represents SSH on the same day. Only SSH values within the range identified as suitable by ModelPAM are plotted (between −1.4 to −1.3m [see Figure 5]; areas beyond this range are shown in white). The estimated location of the SIE on this day is shown as a dashed line on both maps. SSH was always among the two most important predictors (Figure 4). This figure to the right can (partially) explain the pattern of suitable habitats in the left figure: the low band of HS between 60°S and 62°S (overlapping with the location of Weddell Gyre) and areas in the northern part of the study area (to the north of the sbACC), which have SSH values beyond the suitable range for ABWs (as identified by ModelPAM).
Figure S19: Antarctic blue whale PAM data using RL threshold ≥100 dB. Panels A and B show the temporal distribution of acoustic presences. Panels C and D show values of SIC and distance to SIE, respectively, at ABW acoustic detections, using the same RL threshold. Panel E shows marginal response curves for SIC (left) and distance to SIE (right) using different values of RL (100-115 dB). In panel E, different models were fit using the respective acoustic presences without cross-validation. A low RL threshold (~larger detection ranges) resulted in larger sample size (compare A with Figure 2 and B with Figure S1b), covering a much broader range of SIC values and distance to the SIE (compare C-D with Figure S4). The use of small RL threshold (i.e., long distances) led to higher HS at high SIC and at locations much south (c.a. 1000km) of the SIE. This figure highlights the importance of the careful choice of RL threshold and the resulted environmental mismatch of considering calls at much long distances from the recording sites as presences. More details on the recorders are shown in Table 1 and Figures 1-2. Appendix 1
Sources of visual sightings data
• Burkhardt, Elke (2011): Whale sightings during POLARSTERN cruise ANT-XXVII/2. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.760340 • Burkhardt, Elke (2012): Whale sightings during POLARSTERN cruise ANT-XXVII/3. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.783806 • Burkhardt, Elke (2013): Whale sightings during POLARSTERN cruise ANT-XXVIII/2. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819861 • Burkhardt, Elke (2013): Whale sightings during POLARSTERN cruise ANT-XXIX/2. Alfred Wegener Institute, Helmholtz Center for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819866 • Burkhardt, Elke (2020): Whale sightings during POLARSTERN cruise PS96 (ANT-XXXI/2). Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.923113 • Cheeseman, T. and K. Southerland. (2021). Happywhale encounters. • Happywhale (2021) Happywhale - Blue Whale in South Atlantic Ocean. Data downloaded from OBIS-SEAMAP (http://seamap.env.duke.edu/dataset/1759) and originated from Happywhale.com. • Happywhale (2021) Happywhale - Blue Whale in Southern Ocean. Data downloaded from OBIS-SEAMAP (http://seamap.env.duke.edu/dataset/1760) and originated from Happywhale.com. • Herr, Helena; Siebert, Ursula (2018): Aerial cetacean survey Southern Ocean 2012/2013. PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.894914 • Herr, Helena; Viquerat, Sacha; Siebert, Ursula (2018): Ship based cetacean survey Southern Ocean 2014/2015. PANGAEA, https://doi.pangaea.de/10.1594/PANGAEA.894873 • iNaturalist contributors, iNaturalist (2022). iNaturalist Research-grade Observations. iNaturalist.org. Occurrence dataset https://doi.org/10.15468/ab3s5x accessed via GBIF.org. https://www.gbif.org/occurrence/2634034610