Static species distribution models in the marine realm: the case of baleen whales in the Southern Ocean
Aim: Information on the spatiotemporal distribution of marine species is essential for developing proactive management strategies. However, sufficient information is seldom available at large spatial scales, particularly in polar areas. The Southern Ocean (SO) represents a critical habitat for various species, particularly migratory baleen whales. Still, the SO’s remoteness and sea ice coverage disallow obtaining sufficient information on baleen whale distribution and niche preference. Here, we used presence-only species distribution models to predict the circumantarctic habitat suitability of baleen whales and identify important predictors affecting their distribution.
Location: The Southern Ocean (SO)
Methods: We used Maxent to model habitat suitability for Antarctic minke, Antarctic blue, fin, and humpback whales. Our models employ extensive circumantarctic data and carefully prepared predictors describing the SO’s environment and two spatial sampling bias correction options. Species-specific spatial-block cross-validation was used to optimise model complexity and for spatially-independent model evaluation.
Results: Model performance was high on cross-validation, with generally little predicted uncertainty. The most important predictors were derived from sea ice, particularly seasonal mean and variability of sea ice concentration and distance to the sea ice edge.
Main conclusions: Our models support the usefulness of presence-only models as a cost-effective tool in the marine realm, particularly for studying the migratory whales’ distribution. However, we found discrepancies between our results and (within) results of similar studies, mainly due to using different species data quality and quantity, different study area extent, and methodological reasons. We further highlight the limitations of implementing static distribution models in the highly dynamic marine realm. Dynamic models, which relate species information to environmental conditions contemporaneous to species occurrences, can predict near-real-time habitat suitability, necessary for dynamic management. Nevertheless, obtaining sufficient species and environmental predictors at high spatiotemporal resolution, necessary for dynamic models, can be challenging from polar regions.
See also:Explore the distribution of baleen whales in the Southern Ocean (shiny app) Supporting Information for Antarctic minke whales (HTML) Supporting Information for Antarctic blue whales (HTML) Supporting Information for fin whales (HTML) Supporting Information for humpack whales (HTML) Other supporting Information (HTML)
Keywords: Antarctic blue whale, Antarctic minke whale, baleen whales, fin whale, humpback whale, Maxent, presence-only models, Southern Ocean, species distribution models, static species distribution models.
Introduction Information on marine species’ spatiotemporal distribution and their relationship to the environment is pivotal for well-informed, proactive management strategies and conservation actions (Becker et al., 2016; Guisan et al., 2013). However, obtaining sufficient data on marine mammal distribution across large spatial scales is challenging due to financial and logistic constraints, particularly in remote oceans (Kaschner et al., 2012; Robinson et al., 2011). Marine mammal occurrence data are frequently biased towards coastal areas and shallow waters (Robinson et al., 2011) or, for polar regions, to easy-to-access regions during summer months. Species distribution models (SDMs) are empirical methods that relate information on species occurrence to environmental variables to predict potential species distribution and identify potential ecological factors governing their distribution (Phillips et al., 2006). SDMs are promising to further our limited knowledge of marine mammals’ distribution and support marine conservation prioritisation, e.g., identify biologically important areas (Guisan et al., 2013; Redfern et al., 2006; Smith et al., 2020). Although SDMs in marine environments are relatively less common compared to their application in the terrestrial realm, recent years showed a significant increase in SDM usage for marine habitats (Marshall et al., 2014; Melo-Merino et al., 2020; Redfern et al., 2006; Robinson et al., 2011). The main challenge to model the distribution of marine species is the availability of sufficient reliable species data (Dambach & Rödder, 2011; Robinson et al., 2011). Two species information types are commonly used in SDMs: presence-absence and presence-only data. Presence-absence models (e.g., generalised additive models – GAMs) require carefully-designed surveys and thus are more common in small-scale SDM studies (e.g., de Stephanis et al., 2008; Esteban et al., 2013). Absence data is hard to estimate correctly (Lobo et al., 2010), especially for highly mobile and species and from remote areas (Smith et al., 2020). Marine mammals spend a vast amount of time submerged and can be visually detected only when on or near the water surface. Their detection is sensitive to species behaviour and oceanographic and meteorological conditions (Barlow et al., 2001). This imperfect detection can lead to false absences, which affect SDMs evaluation and bias species distribution inferences (Guillera-Arroita, 2017; Lobo et al., 2010). This is even more serious as the detectability of marine mammals varies in time and space (Guillera-Arroita, 2017). Furthermore, even dedicated surveys typically provide only a snapshot of species distribution and represent only a limited time and space range (Kaschner et al., 2006). Hence, not surprisingly, most SDMs use presence-only data. Presence-only models contrast species occurrences to a large sample of background locations to characterize the environment throughout the study area. Recent literature demonstrates the statistical validity of only a few presence-only SDM algorithms, including point process models and Maxent (Renner et al., 2015). The implementation of robust presence-only SDMs is particularly advantageous in the marine realm due to the difficulty of efficiently obtaining systematic presence-absence data (Smith et al., 2020). The Southern Ocean (SO) is a biodiversity hotspot area, showing distinctive biogeographic features and high environmental variability (Convey et al., 2014; De Broyer et al., 2014; Fabri-Ruiz et al., 2019; Guillaumot et al., 2020). The SO’s sea-ice environment represents a critical habitat for many threatened migratory and resident species, particularly for baleen whales (Filun et al., 2020; Thomisch et al., 2016; Van Opzeeland et al., 2013). Nevertheless, research efforts in the SO were limited because of its remoteness, vastness, and sea ice coverage, posing considerable financial and logistical constraints (Bombosch et al., 2014; Scheidat et al., 2011). Our knowledge of the biodiversity in most SO areas seems to reflect sampling effort rather than the actual biodiversity status (Convey et al., 2014), and thus improving sampling effort deserves a high priority for Antarctic science (Guillaumot et al., 2018). Spatiotemporal information on species distributions from the SO, necessary for conservation planning and management, is particularly patchy. Research efforts are generally biased towards relatively small areas of the SO (e.g., the West Antarctic Peninsula), repetitive ship tracks (e.g., to and from Antarctic stations), and mainly limited to summer months. Simultaneously, deep-sea and remote regions (e.g., the Bellingshausen and Amundsen Seas) remain largely underinvestigated (De Broyer et al., 2014). Most research vessels that operate in the SO are biased towards the operationally safe ice-free water and do not engage in the risk and costs of going deep into the sea ice (Herr et al., 2019; Williams et al., 2014), rendering modelling species distribution in the SO challenging (Guillaumot et al., 2018; Guillaumot et al., 2020). Nevertheless, carefully implemented and evaluated presence-only SDMs can be a cost-effective tool to study species potential distribution and habitat and planning for future surveys in the SO. In the SO, several baleen whale species have been extensively hunted to near extension levels during the 20th-century commercial whaling, particularly Antarctic blue and fin whales (Kennicutt et al., 2016; Tulloch et al., 2018). Populations recovery is generally incomplete and shows variant recovery rates between species and SO regions, with some species exhibiting high recovery rates (e.g., humpback whales, Megaptera novaeangliae; Friedlaender et al., 2011; Tulloch et al., 2018) while others remain highly threatened (e.g., Antarctic blue whales, B. musculus intermedia; Branch et al., 2004; Tulloch et al., 2018). Information on the ecology and distribution of baleen whales in the SO is pivotal for the International Whaling Commission’s conservation efforts and measures addressing potential climate change impacts in polar ecosystems (Williams et al., 2014). However, such information is limited (Leaper & Miller, 2011); and thus, relatively few studies have modelled the distribution of baleen whales in the SO. Some species receive more attention, e.g., humpback whales, while others, e.g., fin and Antarctic blue whales, receive less attention (Širović & Hildebrand, 2011). The focus of this paper is to model the circumantarctic distribution of four baleen whale species that feature sufficient sighting data: Antarctic minke whale (AMW, Balaenoptera bonaerensis); Antarctic blue whale (ABW); fin whale (FW, B. physalus); and humpback whale (HW). We performed a rigorous screening of baleen whale circumpolar distribution data in the SO. We used Maxent (Phillips et al., 2006) as it is appropriate for the available presence-only data, with two ways of handling spatial sampling bias (no correction versus rarefication). We used spatial-block cross-validation for independent model evaluation and optimising model complexity to improve predictions. For each species, we predicted its circumantarctic habitat suitability and identified the most important predictors affecting their distribution and species suitability response to environmental changes. We compared our results with previous studies on these species in the SO and discuss reasons for observed differences. Finally, we evaluate the potential limitations of implementing static SDMs in the highly dynamic SDMs.
Methods Species data Cetacean sightings south of 45°S were compiled from different sources. Only sightings after 1980 were considered to maintain a reasonable temporal match between environmental predictors and sightings. Data from three biodiversity repositories were quality controlled: the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/), the Ocean Biodiversity Information System (OBIS, 2018), and OBIS-SEAMAP (Halpin et al., 2009). Other data sources include SO GLOBEC (2001-2002; http://www.ccpo.odu.edu/Research/globec_menu.html), SOWER cruises (https://iwc.int/sower; 2009-2010), RV Polarstern expeditions (https://www.awi.de/expedition/schiffe/polarstern.html), and data published in PANGAEA (https://www.pangaea.de/; details in Appendix 1-5). Data on baleen whales with sufficient sightings (AMW, ABW, FW, and HW) were subjected to further quality control. We excluded erroneous occurrences or those with high uncertainty, e.g., GBIF occurrences flagged with ‘known geospatial issues’ and ‘possible’ certainty level for Polarstern data. As biodiversity data repositories compile data from various sources, the same sighting can be duplicated within or between repositories. We excluded occurrences explicitly duplicated within and between data sources to avoid spurious high relative occurrence rates: only one instance of sightings with identical coordinates and date was retained. We excluded telemetry and catch data to avoid highly correlated occurrences, spatially or temporally. The final dataset consists of ~32 thousand sightings. The temporal distribution of species-specific sightings is shown in Figures S2, S7, S12, and S17. Note that figures in the Supporting Information are grouped by species (Figures S1-20). Environmental predictors Potential predictors were obtained at the highest available spatial and temporal resolution (Table S1). We prepared ecologically-relevant predictors summarising environmental conditions in the SO and act as a proxy for prey availability (Redfern et al., 2006). We calculated monthly and seasonal mean and standard deviation of each dynamic predictor to explore temporal trends and intra-seasonal variability, respectively. Seasons were determined as three-month intervals from January, except for metrics representing sea ice (see below). Bathymetry data were downloaded from GEBCO (Weatherall et al., 2015). From bathymetry, we derived slope, aspect, and closest distances to coast, 500m, and 1000m isobaths. The Antarctic coast was defined as the ice shelf edge, i.e., excluding any cavities under the ice shelves. The 1000m isobath was used to represent the location of the continental shelf break. Chlorophyll-a concentrations (Chl-a) were downloaded as 8-day composites from OCCCI (2002-2017; Sathyendranath et al., 2018). We only considered Chl-a mean and standard deviation in summer, as the spatial coverage in other seasons was rather poor, prohibiting the calculation of meaningful circumpolar averages. Daily absolute dynamic topography (sea surface height, SSH) was obtained from Copernicus (https://copernicus.eu/; 1993-2017), following Bombosch et al. (2014), from which daily (current) speed was estimated. We found only little inter-annual variability of SSH and speed, persuading us to use the annual mean and standard deviation of the whole period. Temperature and salinity data at five standard depths (surface, 100m, 200m, 500m, and 1000m) were obtained from the World Ocean Atlas (1981-2010; Locarnini et al., 2018; Zweng et al., 2018). Daily sea ice concentration (SIC) was obtained from Spreen et al. (2008). We used SIC data for complete years (2003-2010 and 2013-2017), with seasons customised according to the major phases of annual sea ice extent (https://seaice.uni-bremen.de/sea-ice-concentration/time-series/): season 1 (January-March, summer, lowest extent); season 2 (April, sea ice formation start); season 3 (May-November, high extent); and season 4 (December, high sea ice melting). We determined the closest distance to seasonally averaged sea ice edge (SIE), where SIE was identified as the largest polygon with mean SIC >15% (Parkinson, 2002). We assigned a value of zero to cells intersecting with SIE, positive values north of SIE (open water; SIC <15%), and negative values south of SIE (SIC >15%) (following Ainley et al., 2004). All predictors were projected into equal-area projection at 10×10 km resolution. All analyses were restricted to south of the climatological location of the Polar Front as defined by Orsi et al. (1995), which was chosen as a natural boundary of the SO with rather homogeneous hydrographic conditions south of it. Spatial gaps were interpolated using ordinary Kriging (Wackernagel, 1995) when necessary. After the rejection of less-informative predictors, as based on their temporal trends and personal experience, the initial list of predictors included 32 predictors (Table S1b). We implemented predictor transformation when necessary (e.g., square root) to avoid the effect of few extreme values on model stability (Dormann & Kaschner, 2010). We excluded highly correlated predictors by maintaining a moderate maximum variance inflation factor of 4.5 (Zuur et al., 2010). This approach resulted in a total of 15 predictors used in the models (Figures S21-22 and Table 1). Figure S23 shows environmental conditions at species-specific sightings against their full range in the study area. Species distribution models We used Maxent v3.4.1 (Phillips et al., 2017) to train two model sets: 1) using all occurrences to estimate habitat suitability under the point-process modelling framework (following: Renner et al., 2015; ModelAll); and 2) using only one occurrence per cell (ModelUnique). The latter is a special case of rarefaction, a commonly used method to correct for sampling bias and diminish the effect of spatial autocorrelation (Aiello-Lammens et al., 2015). It is expected that bias correction can lead to broader areas of suitable habitats (El-Gabbas & Dormann, 2018a; Phillips et al., 2009). Here, we used both models not to quantify the effect of sampling bias corrections, but to investigate if and how they would affect our conclusions, under the assumption that differences in results reflect on model stability. We used a 5-fold spatial-block cross-validation to evaluate model performance by maintaining spatial independence between training and testing dataset and to reduce the effect of spatial autocorrelation (Roberts et al., 2017). We determined block size and how to distribute blocks into cross-validation folds using blockCV R-package (Valavi et al., 2019): size was determined as median spatial autocorrelation range of environmental conditions at sighting locations; blocks were distributed into folds balancing the number of occurrences (Figure S24). To improve model performance, we tuned Maxent’s parameters using cross-validation (Merow et al., 2013). We used ENMeval R-package (Muscarella et al., 2014) to estimate the best combination of feature classes (transformation of predictors) and regularization multiplier (model complexity). For each model type and species, we used 40 combinations: five feature classes (L/LQ/H/LQH/LQHP; where ‘L’ linear, ‘Q’ quadratic, ‘H’ hinge, and ‘P’ product transformation) and eight regularization multiplier values (0.5 to 4, with 0.5 increment). The combination with highest testing AUC (area under the ROC curve) using cross-validation was used in the final models (Table S2). We present the mean habitat suitability along with the coefficient of variation (ratio between standard deviation and mean prediction) as a measure of predictive uncertainty. In addition to cross-validation, we ran full models that used all occurrences. In each model, we estimated predictor importance using permutation importance and jackknifing. We show the results of the full models in the main text and cross-validated models in the Supporting Information.
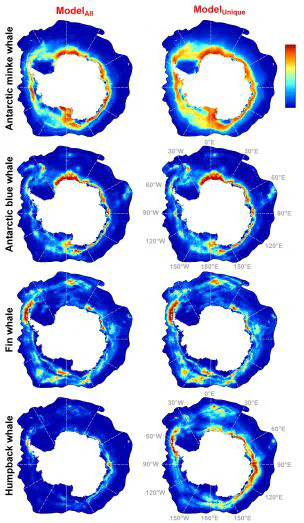
Results In general, both model types (ModelAll and ModelUnique) give similar results, with ModelUnique resulted in a broader range of suitable habitats and slightly lower testing AUC, as expected after bias correction (Figure 1 and Table S2). Generally, the most important predictors were sea ice related (Figure 2). The uncertainty of cross-validated predictions was generally low and did not show a pronounced spatial pattern, reflecting the stability of these sub-models. Antarctic minke whale Models predicted a circumantarctic habitat of AMW, with a general preference closer to the Antarctic coast except for a small patch southwest of the Balleny Islands and the Amundsen Sea coast towards the Ross Sea . Most of the southern part of the Weddell Sea was predicted less suitable (Figures 1 and S1). The most important predictors were distance to summer SIE, mean summer SIC, and SIC variability (Figures 2 and S3). AMW was shown to prefer locations close to SIE and moderate SIC (<50%; Figures S4-5). Antarctic blue whale Suitable areas for ABW were near the Antarctic coast (yet 50-300km offshore), ranging from 30°W eastwards to 170°W (Figures 1 and S6), i.e., along the East Antarctic coast and notably rather sparsely off West Antarctica. Other suitable areas include small patches in the Bellingshausen and Amundsen Seas and between Elephant and the South Sandwich Islands. The most important predictors were SIC variability, mean summer SIC, and distance to 1000m isobath (Figures 2 and S8). Other relatively important predictors were bathymetry, temperature at 200m, and distance to summer SIE. Suitable habitats were predicted in areas with high SIC variability in December (c.a. 35-45%) and low mean summer SIC (<40%) or low to moderate distance to 1000m isobath (<250km; Figure S9-10). ABW habitat is more suitable close to SIE (with lower suitability south of it), at high temperature at 200m (3-5 °C), and locations with moderate depths (3500-4500m; Figure S9). Fin whale The most suitable areas for FW extend eastwards from Elephant Island to South Georgia Island, near Bouvet Islands, small patches close to the Antarctic coast from 30°E eastwards to 180°E, and offshore of the Ross Sea (Figures 1 and S11). Important predictors were distance to summer SIE, mean summer SIC, SIC variability, distance to coast, and SSH variability (Figures 2 and S13). Highest suitability was shown north of the SIE (<200km and at ~1500km from it, only <100km from the coast) or locations with low SIC (<50%), low temperature at 200m (<–1.5 °C), or low SSH variability (Figure S14-15). Humpback whale The effect of sampling bias correction on predicted distribution was most evident for HW, due to intensive sampling west of the Antarctic Peninsula and in East Antarctica. Generally, suitable areas are the Western Antarctic Peninsula eastwards to the South Orkney Islands, around the South Sandwich and Bouvet Islands, and a strip close to the coast from 15°W eastwards to 170°W (Figures 1 and S16). The most important predictors were distance to summer SIE, SIC variability from April to November, and summer SIC. Other important predictors include distance to coast, distance to 1000m isobath, and SSH variability (Figures 2 and S18). HW suitability was higher at locations close to SIE at summer SIC <60%. On the open water side of SIE, high suitability was found only at locations with high SIC variability (Figures S19-20). Moderate suitability is predicted <300km from 1000m isobath and locations close (<100km) or far (>1000km) from the coast (Figure S19).
Discussion Baleen whale habitats in the Southern Ocean Overall, the most important predictors affecting baleen whales’ habitat suitability in the SO are those derived from SIC. Sea ice cover varies within and between years, and this variability plays an integral role in whale distribution (Thiele et al., 2004). Our use of seasonal variability of SIC can be considered as a proxy for site accessibility for whales; the higher the SIC standard deviation, the more accessible for whales (Wege et al., 2020). SIC variability affects prey (krill) survival, population dynamics, and abundance (Fraser & Hofmann, 2003; Thiele et al., 2004), with highest observed abundances close to the SIE (Brierley et al., 2002; Murase et al., 2002; Thiele et al., 2004). Obtaining reliable data on the distribution and abundance of prey, particularly krill, is currently not possible at the circumantarctic scale (Robinson et al., 2011), rendering most studies dependent on remotely-sensed predictors as a proxy for prey availability (Herr et al., 2019). Transition zones, e.g., SIE and continental shelf break, are known high-productivity areas (Beekmans et al., 2010). The use of predictors describing distance to them can serve as a proxy for prey availability. The majority of visual observation data available to us were recorded using vessels unsuited for penetrating the ice, except a few sightings obtained from icebreaker vessels (e.g., Polarstern) and icebreaker-supported helicopter surveys (e.g., Herr et al., 2019). This explains why only little sightings came from the south of the SIE. Nevertheless, distance to SIE was one of the most important predictors for the models of the four study species. In the following, we briefly compare our species-specific results with results of other studies (summarised in Tables 2 and S3) to evaluate the models’ reliabilities in general.
- Antarctic minke whale: Although AMWs are thought to be the most abundant cetacean species in the SO (Williams et al., 2014), they are among the least studied marine mammal populations (Risch et al., 2019). AMWs have a circumantarctic distribution and are considered the major consumer of Antarctic krill in the SO (Beekmans et al., 2010; Kasamatsu et al., 2000b; Williams et al., 2014). Highest AMW density was estimated in the Western Antarctic Peninsula and the Weddell and Ross Seas (Dominello & Širović, 2016; Risch et al., 2019). The AMW is a year-round resident in the SO and occurs throughout a wide range of SIC (Filun et al., 2020; Friedlaender et al., 2011; Herr et al., 2019; Thiele et al., 2004), preferring the SIE area (Dominello & Širović, 2016; Herr et al., 2019; Kasamatsu et al., 2000a; Scheidat et al., 2011; Williams et al., 2014). It has been observed both within the pack ice region and in open water (although in lower numbers) (Beekmans et al., 2010; Ensor, 1989; Friedlaender et al., 2006; Herr et al., 2019; Thiele & Gill, 2004; Williams et al., 2014). They can exploit pack ice and forage krill through sea ice, which is mostly unavailable to other baleen whales, due to their compact small-sized body, hard and pointed nostrum, and high maneuverability (Ainley et al., 2012; Friedlaender et al., 2014). Accordingly, we found high importance of SIC-derived predictors, particularly distance to summer SIE and summer SIC. Sea ice is an essential habitat for AMW and affects their distribution and foraging behaviour in the SO (Friedlaender et al., 2014; Herr et al., 2019; Kasamatsu et al., 2000a; Risch et al., 2019). Thus, with potential future climate change being expected to affect Antarctic krill population dynamics, the AMW’s suitable habitats will shrink throughout the SO (Ainley et al., 2012; Herr et al., 2019; Risch et al., 2019; Williams et al., 2014). Our models show high predicted habitat suitability at SIC up to 50% and low at higher SIC values. Bombosch et al. (2014) showed that AMW habitat suitability was consistently predicted in sea ice-covered areas in the SO. Ainley et al. (2012) showed a consistent positive effect of sea ice cover on AMW suitability, and similarly, Filun et al. (2020) found a strong positive correlation between SIC and AMW acoustic presence in the Weddell Sea, with the highest acoustic activity occurring at SIC >75%. Distance to SIE was also important in other SDM studies with highest suitability close to it (Beekmans et al., 2010; Bombosch et al., 2014; Friedlaender et al., 2011; Herr et al., 2019; Kasamatsu et al., 2000a; Murase et al., 2013; Williams et al., 2014). In contrast, Filun et al. (2020) reported very little acoustic activity near the SIE area in the Weddell Sea during December and January. Some studies discussed the important role of the Antarctic ice shelf break: higher suitability closer to it with a strong decline with increasing distance (Ainley et al., 2012; Beekmans et al., 2010; Herr et al., 2019; Murase et al., 2013). In our model, distance to 1000m isobath had very low importance, although with a similar (albeit weak) relationship pattern. We found low importance of bathymetry (negative relationship), distance to coast, slope, Chl-a (negative relationship), and positive relationship for salinity and water temperature at 200m, but see Kasamatsu et al. (2000a), Friedlaender et al. (2011), Ainley et al. (2012), and Murase (2014) for contradicting results.
- Antarctic blue whale: ABW was once an abundant species in the SO, but is currently extremely rare after its intensive exploitation during the whaling industry era from 1904 until 1978 (Branch et al., 2007; Double et al., 2015; Kasamatsu, 1988; Miller et al., 2015). After the ceasing of the whaling industry, the circumpolar ABW abundance was reported to be depleted to only less than 1% of its original abundance before whaling (Branch et al., 2004; Branch et al., 2007), making the ABW one of the most endangered baleen whale species in the SO (Leaper & Miller, 2011). Little is known on the distribution and migration patterns of ABW in the SO and its relationship with krill (Branch et al., 2007; Double et al., 2015; Thomisch et al., 2016). ABWs were visually sampled relatively infrequently in the SO in comparison with other baleen whales (Murase, 2014; Širović & Hildebrand, 2011), but their calls can be accurately detected (Thomisch et al., 2016). This allowed some studies to model ABW distribution using passive acoustic data (e.g., Shabangu et al., 2017; Širović & Hildebrand, 2011). We found the most important predictors are SIC-derived and distance to 1000m isobath. Other SDM studies provide limited information on the effect of sea ice on ABW’s suitability. High ABW habitat suitability was predicted at low SIC (<40%) and close to summer SIE (Figure S9). Similarly, Širović et al. (2004) and Thomisch et al. (2016) reported a negative correlation between sea ice coverage and the number of detected ABW calls in the Western Antarctic Peninsula and the Weddell Sea, respectively. Nevertheless, ABW was also acoustically present in areas with high winter SIC (90%) in the Weddell Sea (Thomisch et al., 2016) and under non-navigable ice conditions in the Ross Sea (Double et al., 2015) This suggests the overwintering of ABW in highly ice-covered areas, potentially in local recurring polynyas (Thomisch et al., 2016). A high encounter rate of ABW near the SIE was also reported by other studies (Branch et al., 2007; Kasamatsu, 1988; Kasamatsu et al., 2000b; Rankin et al., 2005; Širović et al., 2004). We found moderate importance for bathymetry and temperature at 200m. Highest suitability was found at around 5000m and lower elsewhere. Širović and Hildebrand (2011) found that in the Pacific along the Western Antarctic Peninsula acoustic presence is more suitable at greater depths. Similarly, Murase (2014) found a high abundance peak at depth ~4000m, but with an additional peak near 0m, i.e., close to the coast. In contrast, Shabangu et al. (2017) found the least suitability at around 5000m. We found a positive relationship with temperature at 200m, which coincides with results for calling presences by Širović and Hildebrand (2011). In contrast, Kasamatsu et al. (2000b) reported a high encounter rate at lower temperatures, and Shabangu et al. (2017) showed high suitability of calling whales at ~0°C sea surface temperature (SST). We found moderately low importance of distance to coast, SSH (positive relationship), and Chl-a (positive relationship). Similarly, Širović and Hildebrand (2011) found a non-significant relationship between Chl-a and calling ABW off the Western Antarctic Peninsula. In contrast, Shabangu et al. (2017) showed that these predictors were among the most important predictors for call detections: peak suitability close to coast, then sharply declined until ~1000km; low suitability at SSH around –1.5m and high elsewhere, and high suitability at low Chl-a.
- Fin whale: Although FW was the most caught species in the SO during the 20th-century commercial whaling (>718K whales taken), there is limited information on its distribution, abundance, demographics, and environmental variables affecting its ecology (Herr et al., 2016; Santora et al., 2014). A relatively recent estimation of FW population in the SO has shown that it is currently at only 2% of the presumed pre-whaling estimated abundance (Leaper & Miller, 2011). We found that the most important predictors are SIC-derived predictors, distance to coast, SSH variability, and temperature at 200m. We found highest (although moderate) suitability close and far (~1500km) from the coast. In contrast, Williams et al. (2006) found that abundance increases with the distance from coast, with the lowest intensity close to it off the northern Antarctic Peninsula. Santora et al. (2014) reported FW preference for more complex bathymetry off the northern Antarctic Peninsula. Murase (2014) found three abundance peaks at depths of 4500m, 2200m, and 0m, while Williams et al. (2006) reported a low intensity in depths <1000m. However, we found low importance of bathymetry, with two low suitability peaks at around 4500m and near 0m. We found highest suitability at cold water (<–1.5°C). In contrast, Santora et al. (2014) found a positive correlation between SST and FW abundance off the Western Antarctic Peninsula, and similarly, Kasamatsu (1988) and Kasamatsu et al. (2000b) reported a higher encounter rate at warmer temperatures (>1°C). We found low importance of Chl-a with no clear relationship, which conforms with Murase (2014) except at high Chl-a at which high FW abundance was predicted. We found two moderate suitability peaks in open water, either close or far from (~1500km) SIE. In contrast, other studies noted that FWs are rarely reported near the SIE (Širović et al., 2004): Kasamatsu et al. (2000b) found a high encounter rate far from SIE, and, similarly, Scheidat et al. (2011) reported that the FW majority was observed >140km from SIE.
- Humpback whale: Although HWs were highly exploited during the 20th-century whaling industry, with >150,000 caught whales between 1904 and 1966 (Nowacek et al., 2011), the population has been increasing since the cessation of the whaling industry (Friedlaender et al., 2011). HWs are the most common whale species in the Western Antarctic Peninsula area in summer (Scheidat et al., 2011) and seem to be absent from the Ross Sea (Branch, 2011; Leaper & Miller, 2011). This conforms with the areas predicted as suitable habitats by our models (Figures 1 and S16). Important predictors were SIC-derived, distance to coast and 1000m isobath, as well as SSH variability. Highest suitability is predicted at locations with low SIC or locations either close to SIE or far from it on the sea ice-free side (higher). Schall et al. (2020) found a weak correlation between SIC and HW acoustic presence in the Atlantic sector of the SO, while Van Opzeeland et al. (2013) reported HW acoustic presences at high SIC values (>90%) at an Antarctic coastal recording site during winter. Friedlaender et al. (2011) and Thiele et al. (2004) found highest suitability close to SIE in the Western Antarctic Peninsula. Bombosch et al. (2014) found that HW suitable habitats are primarily in ice-free areas and follow the sea ice retreat. There is apparently a lagged effect of sea ice dynamics on the habitat suitability of HW, suggesting that HWs do not actively track the location of SIE recent retreat, but instead the high productivity that occurs up to two months after sea ice melting (Riekkola et al., 2019). Andrews-Goff et al. (2018) found that predictors associated with the marginal ice zone as the main predictors for HW foraging habitat in the Pacific sector of the SO, particularly mean SIC one month prior to HW arrival to the SO, SIC variability two months prior to arrival, and the distance to SIE (highest at ~65 km), while Riekkola et al. (2019) similarly found that distance to SIE two months prior to arrival as an important driver for HW behaviour in the SO. High HW suitability was predicted at areas very close to the coast, which conforms with the frequent sightings of HWs in coastal areas of the Antarctic Peninsula (Dalla Rosa et al., 2008; Nowacek et al., 2004; Thiele et al., 2004) and near the Greenwich Meridian (Van Opzeeland et al., 2013). We found low importance of bathymetry and slope, with moderate suitability in shallow areas (<1000m) and low elsewhere. Similar results were also shown by Murase (2014), while Friedlaender et al. (2011) reported that HW occupies rugged topography around Marguerite Bay, Western Antarctic Peninsula, with bathymetry and slope among the most important predictors. Murase (2014) found highest abundance at low salinity, two peaks at moderate and high SSH, and high Chl-a. Owen et al. (2019) found slope, Chl-a, and SST among the most important predictors for HW foraging behaviour in East Antarctica. In contrast, we found no clear relationship with salinity, Chl-a, and temperature at 200m, and negative with SSH, but neither of them was an important predictor. In concordance to our results, Riekkola et al. (2019) found a negative relationship between HW foraging behaviour in the Pacific sector of the SO and SSH and low importance of speed, while Kasamatsu et al. (2000b) found no relationship between HW density and SST.
Reasons for discrepancies between studies Unambiguously asserting reasons for the discrepancies between the results discussed above is challenging, as we do not know the true preferred niche of these species. Generally, inconsistency can be attributed to data and methodological reasons. Most studies used occurrences from a limited time frame (e.g., from within summer months of 1-2 years) or covered only a small section of the SO, e.g., the northern Antarctic Peninsula (Santora et al., 2014; Williams et al., 2006), the Western Antarctic Peninsula (Friedlaender et al., 2011; Kasamatsu, 1988; Murase et al., 2013; Širović & Hildebrand, 2011; Thiele et al., 2004), East Antarctica (Owen et al., 2019), the Pacific sector of the SO (Andrews-Goff et al., 2018; Riekkola et al., 2019), the Weddell Sea (Filun et al., 2020; Schall et al., 2020; Thomisch et al., 2019; Van Opzeeland et al., 2013; Williams et al., 2014), the Ross Sea (Murase et al., 2013), and the Bellingshausen and Amundsen Seas (Kasamatsu et al., 2000a). The use of spatially or temporally limited sightings and environmental data makes it difficult for these models to capture the full range of species niche (e.g., causing truncated or biased response curves; Barbet-Massin et al., 2010; Thuiller et al., 2004). Although it is technically possible for these models to predict potential distributions at the circumantarctic scale, the necessary extrapolation to novel conditions or new combinations increases prediction uncertainty (Zurell et al., 2012). Contrastingly, this study used circumantarctic visual observation data, covering a wide range of baleen whale suitable environmental conditions (and their combinations) in the SO. To date, only a few studies investigated the distribution and niche characteristics of baleen whales at the circumantarctic scale (e.g., Bombosch et al., 2014; Branch, 2011; Branch et al., 2007), possibly due to challenges obtaining sufficient data. SDM studies at large scales such as the SO assume stationary species-environmental relationships through space and time, i.e., same niche characteristics at smaller areas of the SO or between seasons (Dormann et al., 2012; El-Gabbas & Dormann, 2018b; Osborne et al., 2007). The distribution of baleen whales varies between seasons and spatial divisions of the SO (Riekkola et al., 2019; Thiele et al., 2004). For example, Beekmans et al. (2010) found inconsistent relationships between environmental predictors and AMW density at circumantarctic and regional scales, suggesting that the relationships between AMW and environmental conditions can be best studied at a regional rather than circumantarctic scale. The vast majority of our sightings were made from the end of December to the end of February (Figure S25). This evident temporal bias towards summer months seems inevitable when using only visual observation data. Passive Acoustic Monitoring (PAM), however, has provided ample evidence for the (near-) year-round presence of several species in this area (Filun et al., 2020; Schall et al., 2020; Thomisch et al., 2016; Van Opzeeland & Hillebrand, 2020; Van Opzeeland et al., 2013). Although we attempted to correct for spatial sampling bias using rarefaction, the absence of visual observations from the Weddell Sea has affected model predictability in this area (Figure 1). The integration of other data types in SDMs, e.g., from tagged animals (e.g., Hindell et al., 2020) and PAM, will be able to fill this gap and forward our understanding of year-round niche preferences of these species. Moreover, studies implemented different response types (e.g., presence-only vs presence-absence), modelling techniques (e.g., GAMs vs Maxent), spatial and temporal resolutions, predictors combinations, environmental bias patterns, and data quality and sampling methods. Furthermore, marginal response curves used to describe species response can be deceptive. To estimate a species’ marginal response curve for any predictor, each other predictor is fixed at one value, neglecting the true multi-dimensionality of the environmental space. For example, Maxent uses a predictor-specific mean value at training observations. The response curve shape can be sensitive to the values at which other predictors are fixed, especially when using limited or biased data or correlated predictors. To overcome this caveat, we show pairwise mean habitat suitability in the environmental space of the most important three predictors, while allowing all predictors to vary together (e.g., Figure S5). However, Maxent quantifies permutation importance based on training AUC drop after permutation (Phillips, 2017). Thus, spatiotemporal biases in species data can highly affect this estimate.
Static SDMs in highly dynamic marine environments The majority of SDMs, particularly when covering large spatial scales, including this study, are static. Static models use predictors summarising environmental conditions over long periods (seasonal or annual averages over >10-50 years; e.g., Sbrocco & Barber, 2013), irrespective of the exact time of species sighting (Bateman et al., 2012). They assume species-environment relationships fixed in space and time and that locations with species detections represent suitable year-round habitats, which likely is a rather poor assumption, especially for migratory species (Bateman et al., 2012; Reside et al., 2010). Static models are more appropriate in highly static environments (as is the case for many terrestrial settings) and for modelling less mobile resident species (e.g., plants and lizards). However, the marine environment is immensely dynamic and undergoes significant changes over short periods, which likely affects the distribution of highly mobile species (Fernandez et al., 2017). Static models can neither capture environmental dynamics nor predict near-real-time species distribution necessary for dynamic ocean management. In a dynamic setting, static models can only provide a fictitious representation (in time) of species suitability for the period over which the model is calibrated. To obtain robust SDMs, it is necessary to maintain a spatiotemporal match between species occurrences and environment (dynamic SDMs; Fernandez et al., 2017; Reside et al., 2010). This is particularly important for highly mobile marine species whose distribution is defined by both short- and long-term variations in ocean conditions (Mannocci et al., 2017). In contrast to conventional static models, dynamic SDMs capture the year-round species-environment relationships and allow predicting habitat suitability at finer temporal resolution (day-week-month). The environment in polar regions, particularly the SO, is highly dynamic due to the seasonal waxing and waning of sea ice (Dayton et al., 1994). It hence appears intuitive to use dynamic, rather than static, SDMs to study habitat preference of migratory whales in the SO. However, obtaining many circumantarctic oceanographic variables at fine spatial and temporal resolution is challenging, compromising dynamic models’ feasibility. Many variables are limited to the sea surface and are not available at high temporal resolution (e.g., daily or weekly) (Fernandez et al., 2017). For example, daily or weekly salinity and productivity data is not available from the SO, and daily oceanic temperatures are limited to the water surface. Other variables show inconsistent and incomplete spatial coverage year-round. For example, Chl-a data is highly patchy and limited to summer months, which constrains its use in year-round dynamic models. The unavailability of sufficient, less temporally- and spatially-biased sightings hinders efficient use of dynamic models and can, in part, explain modellers’ preference for static over dynamic models (Milanesi et al., 2020). High spatiotemporal resolution of some environmental predictors became available only recently. For example, daily SIC data used here are available since June 2002, disallowing using sightings before this time (~22K of 32K sightings used here) in comparable dynamic models. Similarly, sightings without a collection date can be used in static but not dynamic models. Averaging (during the calculation of predictors) over highly varying environments can diminish the influence of environmental variability on the model, possibly leading to over- or under-prediction (Zimmermann et al., 2009). We attempted to diminish the impact of temporal mismatch between sightings and environmental conditions by including the environmental temporal variation (standard deviation) where appropriate. Seasonal variability in combinations with means can express extreme conditions and improve models’ predictive power (Zimmermann et al., 2009). Nevertheless, we emphasise that incorporating environmental variability in static SDMs is inevitably insufficient to capture the SO’s high dynamics. For example, although seasonal SIC variability had high importance in our models, it is unfit to determine species preference to sea ice, and its response curve is hard to interpret. Hence, including predictors representing environmental variabilities may improve predictions, but they are fall short of explaining. Further, summarizing some highly dynamic variables can be challenging, even on a seasonal or monthly scale. For example, we estimated distance to seasonal SIE from seasonal mean SIC. However, sea ice cover (and SIE with it) varies at a high temporal (daily) frequency. Therefore, a single line describing long-term average SIE is not a good representative of the true SIE in any season or month (Figures 3 and S26-27). Although we found high importance of SIC and distance to SIE in summer, relating species observations to their concomitant environmental conditions should be of higher priority in SDMs (Figure S28).
Conclusion
In this study, we used presence-only SDMs (Maxent) to model the circumantarctic habitat of four baleen whale species and identified important predictors affecting their distribution in the Southern Ocean. Model performance was high (Table S2), with generally little predicted cross-validated uncertainty. Unsurprisingly, models identified sea ice-derived predictors and distance to continental shelf break as the main predictors. The indispensable role of sea ice in the lives of many Antarctic species, particularly krill-dependent predators, makes whale species sensitive to future changes in the distribution and the dynamics of the sea ice (Herr et al., 2019; Leaper & Miller, 2011; Thiele et al., 2004). Such environmental change signals have already been reported from polar regions, e.g., the warming of the West Antarctic Peninsula area (Gutt et al., 2015; Vaughan et al., 2003), and the predicted shrinkage of sea ice in the Antarctic under all future climate change scenarios (Gutt et al., 2015; Leaper & Miller, 2011; Solomon et al., 2007). This emphasises the need for more studies on the spatiotemporal distribution of baleen whales in the SO to understand the potential impact of climate change on these species. We compared our species-specific results with results of other studies in the SO and provided reasons for results discrepancy, which is generally attributed to the use of different species data quality and quantity, different study area extent, and methodological reasons.
Maxent is known for its high predictive accuracy and considered one of the most frequently used technique in marine SDM studies (Melo-Merino et al., 2020). Our models back the usefulness of presence-only SDMs like Maxent as a cost- effective tool for studying the distribution of migratory whales (e.g., Smith et al., 2020). The current work further supports the pivotal role of crowdsourcing data from biodiversity repositories (e.g., GBIF and OBIS) and circumantarctic dedicated surveys (e.g., SO GLOBEC and SOWER) to strengthen our knowledge about the distribution and niche of migratory whales in less-surveyed oceans (Beekmans et al., 2010). Nevertheless, future surveys should be prioritised towards less studied areas and the pack ice region, especially beyond the summer months. Alternative data sources, such as PAM and from tagged animals, form a useful addition for studying marine mammals’ habitat preferences year-round, but still require work before these data can be integrated. PAM is particularly useful in the SO for detecting rarely visually-sighted species like ABW and covering difficult-to-access areas (e.g., the ice-covered Weddell Sea). PAM data have already been used in SDMs for odontocete species producing clicks which propagate over short distances allowing to use environmental data from the recording sites (e.g., Gallus et al., 2012; Soldevilla et al., 2011). To date, only few applications have included baleen whales of which calls propagate over long distances causing uncertainty in the interpretation of the relationship between whales and the environment due to this potential mismatch in scales (e.g., Širović & Hildebrand, 2011; Stafford et al., 2009). Nevertheless, the use of PAM data in SDMS, particularly for species in polar waters, holds great potential that calls for exploring this further.
References
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., & Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38(5), 541-545. DOI: 10.1111/ecog.01132
Ainley, D. G., Jacobs, S. S., Ribic, C. A., & Gaffney, I. (2004). Seabird distribution and oceanic features of the Amundsen and southern Bellingshausen seas. Antarctic Science, 10(2), 111-123. DOI: 10.1017/s0954102098000169
Ainley, D. G., Jongsomjit, D., Ballard, G., Thiele, D., Fraser, W. R., & Tynan, C. T. (2012). Modeling the relationship of Antarctic minke whales to major ocean boundaries. Polar Biology, 35(2), 281-290. DOI: 10.1007/s00300-011-1075-1
Andrews-Goff, V., Bestley, S., Gales, N. J., Laverick, S. M., Paton, D., Polanowski, A. M., . . . Double, M. C. (2018). Humpback whale migrations to Antarctic summer foraging grounds through the southwest Pacific Ocean. Scientific Reports, 8(1), 12333. DOI: 10.1038/s41598-018-30748-4
Barbet-Massin, M., Thuiller, W., & Jiguet, F. (2010). How much do we overestimate future local extinction rates when restricting the range of occurrence data in climate suitability models? Ecography, 33(5), 878-886. DOI: 10.1111/j.1600-0587.2010.06181.x
Barlow, J., Gerodete, T., & Forcada, J. (2001). Factors affecting perpendicular sighting distances on shipboard line-transect surveys for cetaceans. Journal of Cetacean Research and Management, 3, 201-212.
Bateman, B. L., VanDerWal, J., & Johnson, C. N. (2012). Nice weather for bettongs: using weather events, not climate means, in species distribution models. Ecography, 35(4), 306-314. DOI: 10.1111/j.1600-0587.2011.06871.x
Becker, E., Forney, K., Fiedler, P., Barlow, J., Chivers, S., Edwards, C., . . . Redfern, J. (2016). Moving towards dynamic ocean management: how well do modeled ocean products predict species distributions? Remote Sensing, 8(2). DOI: 10.3390/rs8020149
Beekmans, B. W. P. M., Forcada, J. F., Murphy, E. J., de Baar, H. J. W., Bathmann, U., & Fleming, A. H. (2010). Generalised additive models to investigate environmental drivers of Antarctic minke whale (Balaenoptera bonaerensis) spatial density in austral summer. Journal of Cetacean Research and Management, 11(2), 115-129.
Bombosch, A., Zitterbart, D. P., Van Opzeeland, I., Frickenhaus, S., Burkhardt, E., Wisz, M. S., & Boebel, O. (2014). Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part I: Oceanographic Research Papers, 91, 101-114. DOI: 10.1016/j.dsr.2014.05.017
Branch, T. A. (2011). Humpback whale abundance south of 60°S from three complete circumpolar sets of surveys. Journal of Cetacean Research and Management, 3, 53-69.
Branch, T. A., Matsuoka, K., & Miyashita, T. (2004). Evidence for increases in Antarctic blue whales based on Bayesian modelling. Marine Mammal Science, 20(4), 726-754. DOI: 10.1111/j.1748-7692.2004.tb01190.x
Branch, T. A., Stafford, K. M., Palacios, D. M., Allison, C., Bannister, J. L., Burton, C. L. K., . . . Warneke, R. M. (2007). Past and present distribution, densities and movements of blue whales Balaenoptera musculus in the Southern Hemisphere and northern Indian Ocean. Mammal Review, 37(2), 116-175. DOI: 10.1111/j.1365-2907.2007.00106.x
Brierley, A. S., Fernandes, P. G., Brandon, M. A., Armstrong, F., Millard, N. W., McPhail, S. D., . . . Griffiths, G. (2002). Antarctic krill under sea ice: elevated abundance in a narrow band just south of ice edge. Science, 295(5561), 1890-1892. DOI: 10.1126/science.1068574
Convey, P., Chown, S. L., Clarke, A., Barnes, D. K. A., Bokhorst, S., Cummings, V., . . . Wall, D. H. (2014). The spatial structure of Antarctic biodiversity. Ecological Monographs, 84(2), 203-244. DOI: 10.1890/12-2216.1
Dalla Rosa, L., Secchi, E. R., Maia, Y. G., Zerbini, A. N., & Heide-Jørgensen, M. P. (2008). Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biology, 31(7), 771-781. DOI: 10.1007/s00300-008-0415-2
Dambach, J., & Rödder, D. (2011). Applications and future challenges in marine species distribution modeling. Aquatic Conservation: Marine and Freshwater Ecosystems, 21(1), 92-100. DOI: 10.1002/aqc.1160
Dayton, P. K., Mordida, B. J., & Bacon, F. (1994). Polar marine communities. American Zoologist, 34(1), 90-99. DOI: 10.1093/icb/34.1.90
De Broyer, C., Koubbi, P., Griffiths, H. J., Raymond, B., Udekem d’Acoz, C. d., Van de Putte, A. P., . . . Ropert-Coudert, Y. (Eds.). (2014). Biogeographic Atlas of the Southern Ocean: Cambridge, SCAR.
de Stephanis, R., Cornulier, T., Verborgh, P., Salazar Sierra, J., Gimeno, N. P., & Guinet, C. (2008). Summer spatial distribution of cetaceans in the Strait of Gibraltar in relation to the oceanographic context. Marine Ecology Progress Series, 353, 275-288. DOI: 10.3354/meps07164
Dominello, T., & Širović, A. (2016). Seasonality of Antarctic minke whale (Balaenoptera bonaerensis) calls off the western Antarctic Peninsula. Marine Mammal Science, 32(3), 826-838. DOI: 10.1111/mms.12302
Dormann, C. F., & Kaschner, K. (2010). Where’s the sperm whale? A species distribution example analysis. Retrieved from: http://www.mced-ecology.org/?page_id=355
Dormann, C. F., Schymanski, S. J., Cabral, J., Chuine, I., Graham, C., Hartig, F., . . . Singer, A. (2012). Correlation and process in species distribution models: bridging a dichotomy. Journal of Biogeography, 39(12), 2119-2131. DOI: 10.1111/j.1365-2699.2011.02659.x
Double, M. C., Miller, B. S., Leaper, R., Olson, P., Cox, M. J., Miller, E., . . . O’Driscoll, R. (2015). Cruise report on blue whale research from the NZ/Aus Antarctic Ecosystems Voyage 2015 of the Southern Ocean Research Partnership. Paper SC/66a/SH/7 presented to the IWC Scientific Committeee. Retrieved from: https://iwc.int/
El-Gabbas, A., & Dormann, C. F. (2018a). Improved species-occurrence predictions in data-poor regions: using large-scale data and bias correction with down-weighted Poisson regression and Maxent. Ecography, 41(7), 1161-1172. DOI: 10.1111/ecog.03149
El-Gabbas, A., & Dormann, C. F. (2018b). Wrong, but useful: regional species distribution models may not be improved by range-wide data under biased sampling. Ecology and Evolution, 8(4), 2196-2206. DOI: 10.1002/ece3.3834
Ensor, P. H. (1989). Minke whales in the pack ice zone, East Antarctica, during the period of maximum annual ice extent. Retrieved from: https://iwc.int/
Esteban, R., Verborgh, P., Gauffier, P., Giménez, J., Afán, I., Cañadas, A., . . . de Stephanis, R. (2013). Identifying key habitat and seasonal patterns of a critically endangered population of killer whales. Journal of the Marine Biological Association of the United Kingdom, 94(06), 1317-1325. DOI: 10.1017/s002531541300091x
Fabri-Ruiz, S., Danis, B., David, B., Saucède, T., & Treml, E. (2019). Can we generate robust species distribution models at the scale of the Southern Ocean? Diversity and Distributions, 25(1), 21-37. DOI: 10.1111/ddi.12835
Fernandez, M., Yesson, C., Gannier, A., Miller, P. I., & Azevedo, J. M. N. (2017). The importance of temporal resolution for niche modelling in dynamic marine environments. Journal of Biogeography, 44(12), 2816-2827. DOI: 10.1111/jbi.13080
Filun, D., Thomisch, K., Boebel, O., Brey, T., Sirovic, A., Spiesecke, S., & Van Opzeeland, I. (2020). Frozen verses: Antarctic minke whales (Balaenoptera bonaerensis) call predominantly during austral winter. Royal Society Open Science, 7(10). DOI: 10.1098/rsos.192112
Fraser, W. R., & Hofmann, E. E. (2003). A predator’s perspective on causal links between climate change, physical forcing and ecosystem response. Marine Ecology Progress Series, 265, 1-15. DOI: 10.3354/meps265001
Friedlaender, A. S., Goldbogen, J. A., Nowacek, D. P., Read, A. J., Johnston, D., & Gales, N. (2014). Feeding rates and under-ice foraging strategies of the smallest lunge filter feeder, the Antarctic minke whale (Balaenoptera bonaerensis). The Journal of Experimental Biology, 217(Pt 16), 2851-2854. DOI: 10.1242/jeb.106682
Friedlaender, A. S., Halpin, P. N., Qian, S. S., Lawson, G. L., Wiebe, P. H., Thiele, D., & Read, A. J. (2006). Whale distribution in relation to prey abundance and oceanographic processes in shelf waters of the Western Antarctic Peninsula. Marine Ecology Progress Series, 317, 297-310. DOI: 10.3354/meps317297
Friedlaender, A. S., Johnston, D. W., Fraser, W. R., Burns, J., Patrick N, H., & Costa, D. P. (2011). Ecological niche modeling of sympatric krill predators around Marguerite Bay, Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 58(13-16), 1729-1740. DOI: 10.1016/j.dsr2.2010.11.018
Gallus, A., Dähne, M., Verfuß, U. K., Bräger, S., Adler, S., Siebert, U., & Benke, H. (2012). Use of static passive acoustic monitoring to assess the status of the ‘Critically Endangered’ Baltic harbour porpoise in German waters. Endangered Species Research, 18(3), 265-278. DOI: 10.3354/esr00448
Guillaumot, C., Fabri-Ruiz, S., Martin, A., Eleaume, M., Danis, B., Feral, J. P., & Saucede, T. (2018). Benthic species of the Kerguelen Plateau show contrasting distribution shifts in response to environmental changes. Ecology and Evolution, 8(12), 6210-6225. DOI: 10.1002/ece3.4091
Guillaumot, C., Moreau, C., Danis, B., & Saucède, T. (2020). Extrapolation in species distribution modelling. Application to Southern Ocean marine species. Progress in Oceanography, 188. DOI: 10.1016/j.pocean.2020.102438
Guillera-Arroita, G. (2017). Modelling of species distributions, range dynamics and communities under imperfect detection: advances, challenges and opportunities. Ecography, 40(2), 281-295. DOI: 10.1111/ecog.02445
Guisan, A., Tingley, R., Baumgartner, J. B., Naujokaitis-Lewis, I., Sutcliffe, P. R., Tulloch, A. I., . . . Buckley, Y. M. (2013). Predicting species distributions for conservation decisions. Ecol Lett, 16(12), 1424-1435. DOI: 10.1111/ele.12189
Gutt, J., Bertler, N., Bracegirdle, T. J., Buschmann, A., Comiso, J., Hosie, G., . . . Xavier, J. C. (2015). The Southern Ocean ecosystem under multiple climate change stresses - an integrated circumpolar assessment. Global Change Biology, 21(4), 1434-1453. DOI: 10.1111/gcb.12794
Halpin, P., Read, A., Fujioka, E., Best, B., Donnelly, B., Hazen, L., . . . Hyrenbach, K. D. (2009). OBIS-SEAMAP: the world data center for marine mammal, sea bird, and sea turtle distributions. Oceanography, 22(2), 104-115. DOI: 10.5670/oceanog.2009.42
Herr, H., Kelly, N., Dorschel, B., Huntemann, M., Kock, K. H., Lehnert, L. S., . . . Scheidat, M. (2019). Aerial surveys for Antarctic minke whales (Balaenoptera bonaerensis) reveal sea ice dependent distribution patterns. Ecology and Evolution, 9(10), 5664-5682. DOI: 10.1002/ece3.5149
Herr, H., Viquerat, S., Siegel, V., Kock, K. H., Dorschel, B., Huneke, W. G. C., . . . Gutt, J. (2016). Horizontal niche partitioning of humpback and fin whales around the West Antarctic Peninsula: evidence from a concurrent whale and krill survey. Polar Biology, 39(5), 799-818. DOI: 10.1007/s00300-016-1927-9
Hindell, M. A., Reisinger, R. R., Ropert-Coudert, Y., Huckstadt, L. A., Trathan, P. N., Bornemann, H., . . . Raymond, B. (2020). Tracking of marine predators to protect Southern Ocean ecosystems. Nature, 580(7801), 87-92. DOI: 10.1038/s41586-020-2126-y
Kasamatsu, F. (1988). Distribution of cetacean sightings in the Antarctic: results obtained from the IWC/IDCR minke whale assessment cruises, 1978/79 to 1983/84. International Whaling Commission. Report. 38. United Kingdom. P.449-473.
Kasamatsu, F., Ensor, P., Joyce, G. G., & Kimura, N. (2000a). Distribution of minke whales in the Bellingshausen and Amundsen Seas (60°W–120°W), with special reference to environmental/physiographic variables. Fisheries Oceanography, 9(3), 214-223. DOI: 10.1046/j.1365-2419.2000.00137.x
Kasamatsu, F., Matsuoka, K., & Hakamada, T. (2000b). Interspecific relationships in density among the whale community in the Antarctic. Polar Biology, 23(7), 466-473. DOI: 10.1007/s003009900107
Kaschner, K., Quick, N. J., Jewell, R., Williams, R., & Harris, C. M. (2012). Global coverage of cetacean line-transect surveys: status quo, data gaps and future challenges. PLoS One, 7(9), e44075. DOI: 10.1371/journal.pone.0044075
Kaschner, K., Watson, R., Trites, A. W., & Pauly, D. (2006). Mapping world-wide distributions of marine mammal species using a relative environmental suitability (RES) model. Marine Ecology Progress Series, 316, 285-310. DOI: 10.3354/meps316285
Kennicutt, M. C., Kim, Y. D., Rogan-Finnemore, M., Anandakrishnan, S., Chown, S. L., Colwell, S., . . . Yang, H. (2016). Delivering 21st century Antarctic and Southern Ocean science. Antarctic Science, 28(06), 407-423. DOI: 10.1017/s0954102016000481
Leaper, R., & Miller, C. (2011). Management of Antarctic baleen whales amid past exploitation, current threats and complex marine ecosystems. Antarctic Science, 23(06), 503-529. DOI: 10.1017/s0954102011000708
Lobo, J. M., Jiménez-Valverde, A., & Hortal, J. (2010). The uncertain nature of absences and their importance in species distribution modelling. Ecography, 33(1), 103-114. DOI: 10.1111/j.1600-0587.2009.06039.x
Locarnini, R. A., Mishonov, A. V., Baranova, O. K., Boyer, T. P., Zweng, M. M., Garcia, H. E., . . . Smolyar, I. (2018). World Ocean Atlas 2018, Volume 1: Temperature. A. Mishonov Technical Ed.; NOAA Atlas NESDIS 81, 52 pp. Retrieved from: https://www.nodc.noaa.gov/OC5/woa18/pubwoa18.html
Mannocci, L., Boustany, A. M., Roberts, J. J., Palacios, D. M., Dunn, D. C., Halpin, P. N., . . . Winship, A. J. (2017). Temporal resolutions in species distribution models of highly mobile marine animals: recommendations for ecologists and managers. Diversity and Distributions, 23(10), 1098-1109. DOI: 10.1111/ddi.12609
Marshall, C. E., Glegg, G. A., & Howell, K. L. (2014). Species distribution modelling to support marine conservation planning: the next steps. Marine Policy, 45, 330-332. DOI: 10.1016/j.marpol.2013.09.003
Melo-Merino, S. M., Reyes-Bonilla, H., & Lira-Noriega, A. (2020). Ecological niche models and species distribution models in marine environments: a literature review and spatial analysis of evidence. Ecological Modelling, 415. DOI: 10.1016/j.ecolmodel.2019.108837
Merow, C., Smith, M. J., & Silander, J. A. (2013). A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography, 36(10), 1058-1069. DOI: 10.1111/j.1600-0587.2013.07872.x
Milanesi, P., Della Rocca, F., & Robinson, R. A. (2020). Integrating dynamic environmental predictors and species occurrences: toward true dynamic species distribution models. Ecology and Evolution, 10(2), 1087-1092. DOI: 10.1002/ece3.5938
Miller, B. S., Barlow, J., Calderan, S., Collins, K., Leaper, R., Olson, P., . . . Double, M. C. (2015). Validating the reliability of passive acoustic localisation: a novel method for encountering rare and remote Antarctic blue whales. Endangered Species Research, 26(3), 257-269. DOI: 10.3354/esr00642
Murase, H. (2014). Estimation of circumpolar spatial distributions of baleen whales in the Antarctic in the period of the IWC IDCR/SOWER CPII and CPIII (1994 – 2004). Paper SC/65b/IA10 presented to the 65a IWC Scientific Committee, May 2014. 42 pp. Retrieved from: https://iwc.int/
Murase, H., Kitakado, T., Hakamada, T., Matsuoka, K., Nishiwaki, S., & Naganobu, M. (2013). Spatial distribution of Antarctic minke whales (Balaenoptera bonaerensis) in relation to spatial distributions of krill in the Ross Sea, Antarctica. Fisheries Oceanography, 22(3), 154-173. DOI: 10.1111/fog.12011
Murase, H., Matsuoka, K., Ichii, T., & Nishiwaki, S. (2002). Relationship between the distribution of euphausiids and baleen whales in the Antarctic (35°E – 145°W). Polar Biology, 25(2), 135-145. DOI: 10.1007/s003000100321
Muscarella, R., Galante, P. J., Soley-Guardia, M., Boria, R. A., Kass, J. M., Uriarte, M., . . . McPherson, J. (2014). ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution, 5(11), 1198-1205. DOI: 10.1111/2041-210x.12261
Nowacek, D. P., Friedlaender, A. S., Halpin, P. N., Hazen, E. L., Johnston, D. W., Read, A. J., . . . Zhu, Y. (2011). Super-aggregations of krill and humpback whales in Wilhelmina Bay, Antarctic Peninsula. PLoS One, 6(4), e19173. DOI: 10.1371/journal.pone.0019173
Nowacek, D. P., Johnson, M. P., & Tyack, P. L. (2004). North Atlantic right whales (Eubalaena glacialis) ignore ships but respond to alerting stimuli. Proceedings of the Royal Society B: Biological Sciences, 271(1536), 227-231. DOI: 10.1098/rspb.2003.2570
OBIS. (2018). Ocean Biogeographic Information System. Intergovernmental Oceanographic Commission of UNESCO. https://www.iobis.org.
Orsi, A. H., Whitworth, T., & Nowlin, W. D. (1995). On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Research Part I: Oceanographic Research Papers, 42(5), 641-673. DOI: 10.1016/0967-0637(95)00021-W
Osborne, P. E., Foody, G. M., & Suárez-Seoane, S. (2007). Non-stationarity and local approaches to modelling the distributions of wildlife. Diversity and Distributions, 13(3), 313-323. DOI: 10.1111/j.1472-4642.2007.00344.x
Owen, K., Jenner, K. C. S., Jenner, M. N. M., McCauley, R. D., & Andrews, R. D. (2019). Water temperature correlates with baleen whale foraging behaviour at multiple scales in the Antarctic. Marine and Freshwater Research, 70(1), 19-32. DOI: 10.1071/Mf17288
Parkinson, C. L. (2002). Trends in the length of the Southern Ocean sea-ice season, 1979–99. Annals of Glaciology, 34, 435-440. DOI: 10.3189/172756402781817482
Phillips, S. J. (2017). A Brief Tutorial on Maxent. Retrieved from: http://biodiversityinformatics.amnh.org/open_source/maxent/. Accessed on August 2020.
Phillips, S. J., Anderson, R. P., Dudik, M., Schapire, R. E., & Blair, M. E. (2017). Opening the black box: an open-source release of Maxent. Ecography, 40(7), 887-893. DOI: 10.1111/ecog.03049
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190(3-4), 231-259. DOI: 10.1016/j.ecolmodel.2005.03.026
Phillips, S. J., Dudik, M., Elith, J., Graham, C. H., Lehmann, A., Leathwick, J., & Ferrier, S. (2009). Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl, 19(1), 181-197. DOI: 10.1890/07-2153.1
Rankin, S., ljungblad, D., Clark, C., & Kato, H. (2005). Vocalisations of Antarctic blue whales, Balaenoptera musculus intermedia, recorded during the 2001/2002 and 2002/2003 IWC/SOWER circumpolar cruises, Area V, Antarctica. Journal of Cetacean Research and Management, 7(1), 13-20.
Redfern, J. V., Ferguson, M. C., Becker, E. A., Hyrenbach, K. D., Good, C., Barlow, J., . . . Werne, F. (2006). Techniques for cetacean–habitat modeling. Marine Ecology Progress Series, 310, 271-295. DOI: 10.3354/meps310271
Renner, I. W., Elith, J., Baddeley, A., Fithian, W., Hastie, T., Phillips, S. J., . . . O’Hara, R. B. (2015). Point process models for presence-only analysis. Methods in Ecology and Evolution, 6(4), 366-379. DOI: 10.1111/2041-210x.12352
Reside, A. E., Vanderwal, J. J., Kutt, A. S., & Perkins, G. C. (2010). Weather, not climate, defines distributions of vagile bird species. PLoS One, 5(10), e13569. DOI: 10.1371/journal.pone.0013569
Riekkola, L., Andrews-Goff, V., Friedlaender, A., Constantine, R., & Zerbini, A. N. (2019). Environmental drivers of humpback whale foraging behavior in the remote Southern Ocean. Journal of Experimental Marine Biology and Ecology, 517, 1-12. DOI: 10.1016/j.jembe.2019.05.008
Risch, D., Norris, T., Curnock, M., & Friedlaender, A. (2019). Common and Antarctic Minke whales: conservation status and future research directions. Frontiers in Marine Science, 6. DOI: 10.3389/fmars.2019.00247
Roberts, D. R., Bahn, V., Ciuti, S., Boyce, M. S., Elith, J., Guillera-Arroita, G., . . . Dormann, C. F. (2017). Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography, 40(8), 913-929. DOI: 10.1111/ecog.02881
Robinson, L. M., Elith, J., Hobday, A. J., Pearson, R. G., Kendall, B. E., Possingham, H. P., & Richardson, A. J. (2011). Pushing the limits in marine species distribution modelling: lessons from the land present challenges and opportunities. Global Ecology and Biogeography, 20(6), 789-802. DOI: 10.1111/j.1466-8238.2010.00636.x
Santora, J. A., Schroeder, I. D., & Loeb, V. J. (2014). Spatial assessment of fin whale hotspots and their association with krill within an important Antarctic feeding and fishing ground. Marine Biology, 161(10), 2293-2305. DOI: 10.1007/s00227-014-2506-7
Sathyendranath, S., Grant, M., Brewin, R. J. W., Brockmann, C., Brotas, V., Chuprin, A., . . . Zibordi, G. (2018). ESA Ocean Colour Climate Change Initiative (Ocean_Colour_cci): Version 3.1 Data. Centre for Environmental Data Analysis, 04 July 2018.
Sbrocco, E. J., & Barber, P. H. (2013). MARSPEC: ocean climate layers for marine spatial ecology. Ecology, 94(4), 979-979. DOI: 10.1890/12-1358.1
Schall, E., Thomisch, K., Boebel, O., Gerlach, G., Spiesecke, S., & Van Opzeeland, I. (2020). Large-scale spatial variabilities in the humpback whale acoustic presence in the Atlantic sector of the Southern Ocean. Royal Society Open Science, 7(12). DOI: 10.1098/rsos.201347
Scheidat, M., Friedlaender, A., Kock, K. H., Lehnert, L., Boebel, O., Roberts, J., & Williams, R. (2011). Cetacean surveys in the Southern Ocean using icebreaker-supported helicopters. Polar Biology, 34(10), 1513-1522. DOI: 10.1007/s00300-011-1010-5
Shabangu, F. W., Yemane, D., Stafford, K. M., Ensor, P., & Findlay, K. P. (2017). Modelling the effects of environmental conditions on the acoustic occurrence and behaviour of Antarctic blue whales. PLoS One, 12(2), e0172705. DOI: 10.1371/journal.pone.0172705
Širović, A., & Hildebrand, J. A. (2011). Using passive acoustics to model blue whale habitat off the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 58(13-16), 1719-1728. DOI: 10.1016/j.dsr2.2010.08.019
Širović, A., Hildebrand, J. A., Wiggins, S. M., McDonald, M. A., Moore, S. E., & Thiele, D. (2004). Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 51(17-19), 2327-2344. DOI: 10.1016/j.dsr2.2004.08.005
Smith, J. N., Kelly, N., & Renner, I. W. (2020). Validation of presence-only models for conservation planning and the application to whales in a multiple-use marine park. Ecological Applications, e02214. DOI: 10.1002/eap.2214
Soldevilla, M. S., Wiggins, S. M., Hildebrand, J. A., Oleson, E. M., & Ferguson, M. C. (2011). Risso’s and Pacific white-sided dolphin habitat modeling from passive acoustic monitoring. Marine Ecology Progress Series, 423, 247-260. DOI: 10.3354/meps08927
Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K. B., . . . Miller, H. L. (Eds.). (2007). Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Spreen, G., Kaleschke, L., & Heygster, G. (2008). Sea ice remote sensing using AMSR-E 89-GHz channels. Journal of Geophysical Research, 113(C2). DOI: 10.1029/2005jc003384
Stafford, K. M., Citta, J. J., Moore, S. E., Daher, M. A., & George, J. E. (2009). Environmental correlates of blue and fin whale call detections in the North Pacific Ocean from 1997 to 2002. Marine Ecology Progress Series, 395, 37-53. DOI: 10.3354/meps08362
Thiele, D., Chester, E. T., Moore, S. E., Širovic, A., Hildebrand, J. A., & Friedlaender, A. S. (2004). Seasonal variability in whale encounters in the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 51(17-19), 2311-2325. DOI: 10.1016/j.dsr2.2004.07.007
Thiele, D., & Gill, P. C. (2004). Cetacean observations during a winter voyage into Antarctic sea ice south of Australia. Antarctic Science, 11(01). DOI: 10.1017/s0954102099000073
Thomisch, K., Boebel, O., Bachmann, J., Filun, D., Neumann, S., Spiesecke, S., & Van Opzeeland, I. (2019). Temporal patterns in the acoustic presence of baleen whale species in a presumed breeding area off Namibia. Marine Ecology Progress Series, 620, 201-214. DOI: 10.3354/meps12952
Thomisch, K., Boebel, O., Clark, C. W., Hagen, W., Spiesecke, S., Zitterbart, D. P., & Van Opzeeland, I. (2016). Spatio-temporal patterns in acoustic presence and distribution of Antarctic blue whales Balaenoptera musculus intermedia in the Weddell Sea. Endangered Species Research, 30, 239-253. DOI: 10.3354/esr00739
Thuiller, W., Brotons, L., Araújo, M. B., & Lavorel, S. (2004). Effects of restricting environmental range of data to project current and future species distributions. Ecography, 27(2), 165-172. DOI: 10.1111/j.0906-7590.2004.03673.x
Tulloch, V. J. D., Plaganyi, E. E., Matear, R., Brown, C. J., & Richardson, A. J. (2018). Ecosystem modelling to quantify the impact of historical whaling on Southern Hemisphere baleen whales. Fish and Fisheries, 19(1), 117-137. DOI: 10.1111/faf.12241
Valavi, R., Elith, J., Lahoz-Monfort, J. J., & Guillera-Arroita, G. (2019). blockCV: An r package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods in Ecology and Evolution, 10(2), 225-232. DOI: 10.1111/2041-210x.13107
Van Opzeeland, I., & Hillebrand, H. (2020). Year-round passive acoustic data reveal spatio-temporal patterns in marine mammal community composition in the Weddell Sea, Antarctica. Marine Ecology Progress Series, 638, 191-206. DOI: 10.3354/meps13258
Van Opzeeland, I., Van Parijs, S., Kindermann, L., Burkhardt, E., & Boebel, O. (2013). Calling in the cold: pervasive acoustic presence of humpback whales (Megaptera novaeangliae) in Antarctic coastal waters. PLoS One, 8(9), e73007. DOI: 10.1371/journal.pone.0073007
Vaughan, D. G., Marshall, G. J., Connolley, W. M., Parkinson, C., Mulvaney, R., Hodgson, D. A., . . . Turner, J. (2003). Recent rapid regional climate warming on the Antarctic Peninsula. Climatic Change, 60(3), 243-274. DOI: 10.1023/A:1026021217991
Wackernagel, H. (1995). Ordinary Kriging. In Multivariate Geostatistics. Springer, Berlin, Heidelberg (pp. 74-81).
Weatherall, P., Marks, K. M., Jakobsson, M., Schmitt, T., Tani, S., Arndt, J. E., . . . Wigley, R. (2015). A new digital bathymetric model of the world’s oceans. Earth and Space Science, 2(8), 331-345. DOI: 10.1002/2015ea000107
Wege, M., Salas, L., & LaRue, M. (2020). Citizen science and habitat modelling facilitates conservation planning for crabeater seals in the Weddell Sea. Diversity and Distributions, 26(10), 1291-1304. DOI: 10.1111/ddi.13120
Williams, R., Hedley, S. L., & Hammond, P. S. (2006). Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecology and Society, 11(1).
Williams, R., Kelly, N., Boebel, O., Friedlaender, A. S., Herr, H., Kock, K. H., . . . Brierley, A. S. (2014). Counting whales in a challenging, changing environment. Scientific Reports, 4, 4170. DOI: 10.1038/srep04170
Zimmermann, N. E., Yoccoz, N. G., Edwards, T. C., Jr., Meier, E. S., Thuiller, W., Guisan, A., . . . Pearman, P. B. (2009). Climatic extremes improve predictions of spatial patterns of tree species. PNAS, 106 Suppl 2, 19723-19728. DOI: 10.1073/pnas.0901643106
Zurell, D., Elith, J., & Schröder, B. (2012). Predicting to new environments: tools for visualizing model behaviour and impacts on mapped distributions. Diversity and Distributions, 18(6), 628-634. DOI: 10.1111/j.1472-4642.2012.00887.x
Zuur, A. F., Ieno, E. N., & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1), 3-14. DOI: 10.1111/j.2041-210X.2009.00001.x
Zweng, M. M., Reagan, J. R., Seidov, D., Boyer, T. P., Locarnini, R. A., Garcia, H. E., . . . Smolyar, I. (2018). World Ocean Atlas 2018, Volume 2: Salinity. A. Mishonov Technical Ed.; NOAA Atlas NESDIS 82, 50 pp. Retrieved from: https://www.nodc.noaa.gov/OC5/woa18/pubwoa18.html
Data Accessibility Statement: The dataset analysed during the current study is already publicly available (details in Appendix 1-5). The interactive distribution of cleaned occurrences is available at this web-application: https://awi-oza.shinyapps.io/BaleenWhales/.
Table 1: List of environmental predictors used in the models. Statistics: type of statistics used to calculate each predictor (SD = standard deviation); season: which season or month range was used; transformation: transformations implemented to maximize uniformity of the data; abbreviation: the abbreviation used in the figures; VIF: the value of variance inflation factor. Summer was defined as from January to March. See Table S1 for more information on the predictors used.
Table 2: Summary of the comparison between this study’s results and like studies on the Antarctic minke whale in the SO. Important predictors, as identified by this study, are shaded dark grey in the column header (> 5% permutation importance for the full models, in descending order). Cell colours represent the agreement between our study results and other studies: green, high agreement; orange, some disagreement; red, high disagreement. Empty cells represent situations when the given predictor was not tested. Similar results for other species are shown in Table S3. Abbreviations used: SIC = sea ice concentration; SIE = sea ice edge; SSH = sea surface height; Chl-a = chlorophyll-a concentration; ✓ = similar results; ⊕ = positive relationship; ⊖ = negative relationship; imp. = importance; Dist. = distance; SD = standard deviation; Temp. = water temperature.
Figure 1: Predicted habitat suitability of four baleen whale species in the Southern Ocean using ModelAll (all occurrences, left) and ModelUnique (removed duplicate sightings, right). These maps represent predictions from the respective ‘full model’, calibrated without cross-validation. Mean prediction from cross-validated models and their coefficient of variation are shown in the Supporting Information. Map colours range from blue (low suitability) to red (high suitability). All maps are on Maxent’s cloglog scale.
Figure 2: Permutation importance of environmental predictors used to train the models of (a) Antarctic minke whale; (b) Antarctic blue whale; (c) fin whale; and (d) humpback whale. Results of ModelAll (all occurrences) are shown in dark grey bars, while the results of ModelUnique (removed duplicates) are shown in light grey bars. Bars and their accompanying error bars represent the mean and standard deviation of the permutation importance of cross-validated models. Blue dots represent the permutation importance of full models calibrated without cross-validation. The horizontal dashed line represents 5% permutation importance, above which environmental predictors were considered as potentially important for the distribution of the species (light green-dashed area). Plots for the jackknifing test are shown in the Supporting Information. For more information on the predictors used, see Table 1.
Figure 3: Locations of daily sea ice edge (SIE) in austral summer from 2002 to 2019. The outer black line represents the location of the Southern Ocean Polar Front. The first three maps show how daily SIE varies within each month across years. The last map shows the location of daily SIE in summer months (blue lines) along with the mean summer SIE used in this study (bold black line). The darker the blue, the more often the daily SIE is located. For seasonal and monthly trends, see Figures S26-27. The numbers on the top-left map represent the geographical location of places mentioned in this paper: (1) the Weddell Sea; (2) the Bellingshausen Sea; (3) the Amundsen Sea; (4) the Ross Sea; (5) the Antarctic Peninsula; (6) Elephant Island; (7) the South Orkney Islands; (8) the South Sandwich Islands; (9) South Georgia Island; (10) the Balleny Islands; and (11) Bouvet Island.
Supporting Information for “Static species distribution models in the marine realm: the case of baleen whales in the Southern Ocean” – Diversity and Distributions
Table S1: Environmental predictors preparation
A) List of all initial variables and their derived predictors, unit, original temporal and spatial resolution, and data source. B) List of 32 initially selected predictors based on data visualization and personal experience before excluding highly correlated predictors. The final list of predictors used to run the models is shown in Table 1 of the main text.
Table S2: The results of the cross-validated Maxent models. ModelAll represents models run using all occurrences, while ModelUnique represents models run after removing duplicated occurrences within each 10×10 km cell. Model parameters and testing AUC columns show the best combination of feature classes (where ‘L’ linear, ‘Q’ quadratic, ‘H’ hinge, and ‘P’ product transformation) and regularization multiplier as well as the mean ± standard deviation of the testing AUC on spatial-block cross-validation. Block size represents the width of species- and model-specific spatial blocks. The number of occupied pixels represents the number of pixels (10×10 km) in the Southern Ocean with at least one sighting of each species.
Table S3: Summary of the comparison between this study’s results and like studies on Antarctic blue, fin, and humpback whales in the SO. Important predictors, as identified by this study, are shaded dark grey in the column header (> 5% permutation importance for the full models, in descending order). Cell colours represent the agreement between our study results and other studies: green, high agreement; orange, some disagreement; red, high disagreement. Empty cells represent situations when the given predictor was not tested. Results for Antarctic minke whale are shown in Table 2. Abbreviations used: SIC = sea ice concentration; SIE = sea ice edge; SSH = sea surface height; Chl-a = chlorophyll-a concentration; ✓ = similar results; ⊕ = positive relationship; ⊖ = negative relationship; imp. = importance; Dist. = distance; SD = standard deviation; Temp. = water temperature.
Figure S1: Mean and coefficient of variation of the predicted habitat suitability of the Antarctic minke whale on cross-validation. The top left map shows occurrences used to train the models. The coefficient of variation was calculated as the ratio between standard deviation and mean cross-validated habitat suitability.
Figure S2: Temporal distribution of available occurrences for the Antarctic minke whale. The histogram shows the number of occurrences within each ten-year bin. The red vertical line differentiates occurrences excluded (before 1980) or included (after 1980) in the analysis. The right plot shows the number of occurrences after 1980 on each calendar day. The majority of occurrences was collected during summer months (particularly in January and February).
Figure S3: Results for Maxent’s jackknifing test for ModelAll (left) and ModelUnique (right) for the Antarctic minke whale. Red bars represent the cross-validated mean regularized training gain (a measure of goodness-of-fit) for sub-models calibrated using all predictors except the respective predictor. In contrast, blue bars represent complementary results for models calibrated only with the respective predictor. Error bars represent the standard deviation of the regularized training gain on cross-validation. Grey points show results for the full model, calibrated with no cross-validation. For more information on predictor abbreviations, see Table 1.
Figure S4: Marginal response curves for the Antarctic minke whale models. Response curves describe the cloglog prediction changes as each environmental predictor changes, with keeping all other predictors at their average value at training presences. As these response curves are sensitive to the fixed value of other predictors, we also provide the predicted values in the environmental space of the most important three predictors (see Figure S5). Solid blue lines and dashed area represent the mean and standard deviation of the cross-validated response curve of ModelAll, while the red line and dashed area represents ModelUnique. PI represents the permutation importance of the predictor, with predictors with more than 5% permutation importance shown in a larger font. To make it easy to understand, transformed predictors (distance to 1000m isobath, slope, mean summer chlorophyll, annual variability of sea surface height, and annual mean speed) were also back-transformed for visualization. The top green rug shows values at species occurrences; similarly, the bottom rung shows values south of the Polar Front.
Figure S5: Mean habitat suitability of the Antarctic minke whale in the environmental space of the three most important environmental predictors. The first row shows combinations at species occurrences, while the second and third rows show predictions from ModelAll and ModelUnique, respectively. In each plot in the second and third row, mean habitat suitability at each respective combination was averaged over all available combinations of other predictors. White areas represent no available environmental combinations in the data. Colours range from blue (low prediction) to red (high prediction). For more information on the predictors acronyms used, see Table 1. Results of the Antarctic blue whale (Figures S6-10)
Figure S6: Mean and coefficient of variation of the predicted habitat suitability of the Antarctic blue whale on cross-validation. The top left map shows occurrences used to train the models. The coefficient of variation was calculated as the ratio between standard deviation and mean cross-validated habitat suitability.
Figure S7: The temporal distribution of available occurrences for the Antarctic blue whale. The histogram shows the number of occurrences within each ten-year bin. The red vertical line differentiates occurrences excluded (before 1980) or included (after 1980) in the analysis. The right plot shows the number of occurrences after 1980 on each calendar day. The majority of occurrences was collected during summer months (particularly in January and February).
Figure S8: Results for Maxent’s jackknifing test for ModelAll (left) and ModelUnique (right) for the Antarctic blue whale. Red bars represent the cross-validated mean regularized training gain (a measure of goodness-of-fit) for sub-models calibrated using all predictors except the respective predictor. In contrast, blue bars represent complementary results for models calibrated only with the respective predictor. Error bars represent the standard deviation of the regularized training gain on cross-validation. Grey points show results for the full model, calibrated with no cross-validation. For more information on predictor abbreviations, see Table 1.
Figure S9: Marginal response curves for the Antarctic blue whale models. Response curves describe the cloglog prediction changes as each environmental predictor changes, with keeping all other predictors at their average value at training presences. As these response curves are sensitive to the fixed value of other predictors, we also provide the predicted values in the environmental space of the most important three predictors (see Figure S10). Solid blue lines and dashed area represent the mean and standard deviation of the cross-validated response curve of ModelAll, while the red line and dashed area represents ModelUnique. PI represents the permutation importance of the predictor, with predictors with more than 5% permutation importance shown in a larger font. For better understanding, transformed predictors (distance to 1000m isobath, slope, mean summer chlorophyll, annual variability of sea surface height, and annual mean speed) were also back-transformed for visualization. The top green rug shows values at species occurrences; similarly, the bottom rung shows values south of the Polar Front.
Figure S10: Mean habitat suitability of the Antarctic blue whale in the environmental space of the three most important environmental predictors. The first row shows combinations at species occurrences, while the second and third rows show predictions from ModelAll and ModelUnique, respectively. In each plot in the second and third row, mean habitat suitability at each respective combination was averaged over all available combinations of other predictors. White areas represent no available environmental combinations in the data. Colours range from blue (low prediction) to red (high prediction). For more information on the predictors acronyms used, see Table 1. Results of the fin whale (Figures S11-15)
Figure S11: Mean and coefficient of variation of the predicted habitat suitability of the fin whale on cross-validation. The top left map shows occurrences used to train the models. The coefficient of variation was calculated as the ratio between standard deviation and mean cross-validated habitat suitability.
Figure S12: The temporal distribution of available occurrences for the fin whale. The histogram shows the number of occurrences within each ten-year bin. The red vertical line differentiates occurrences excluded (before 1980) or included (after 1980) in the analysis. The right plot shows the number of occurrences after 1980 on each calendar day. The majority of occurrences was collected during summer months (particularly in January and February).
Figure S13: Results for Maxent’s jackknifing test for ModelAll (left) and ModelUnique (right) for the fin whale. Red bars represent the cross-validated mean regularized training gain (a measure of goodness-of-fit) for sub-models calibrated using all predictors except the respective predictor. In contrast, blue bars represent complementary results for models calibrated only with the respective predictor. Error bars represent the standard deviation of the regularized training gain on cross-validation. Grey points show results for the full model, calibrated with no cross-validation. For more information on predictor abbreviations, see Table 1.
Figure S14: Marginal response curves for the fin whale models. Response curves describe the cloglog prediction changes as each environmental predictor changes, with keeping all other predictors at their average value at training presences. As these response curves are sensitive to the fixed value of other predictors, we also provide the predicted values in the environmental space of the most important three predictors (see Figure S15). Solid blue lines and dashed area represent the mean and standard deviation of the cross-validated response curve of ModelAll, while the red line and dashed area represents ModelUnique. PI represents the permutation importance of the predictor, with predictors with more than 5% permutation importance shown in a larger font. For better understanding, transformed predictors (distance to 1000m isobath, slope, mean summer chlorophyll, annual variability of sea surface height, and annual mean speed) were also back-transformed for visualization. The top green rug shows values at species occurrences; similarly, the bottom rung shows values south of the Polar Front.
Figure S15: Mean habitat suitability of the fin whale in the environmental space of the three most important environmental predictors. The first row shows combinations at species occurrences, while the second and third rows show predictions from ModelAll and ModelUnique, respectively. In each plot in the second and third row, mean habitat suitability at each respective combination was averaged over all available combinations of other predictors. White areas represent no available environmental combinations in the data. Colours range from blue (low prediction) to red (high prediction). For more information on the predictors acronyms used, see Table 1. Results of the humpback whale (Figures S16-20)
Figure S16: Mean and coefficient of variation of the predicted habitat suitability of the humpback whale on cross-validation. The top left map shows occurrences used to train the models. The coefficient of variation was calculated as the ratio between standard deviation and mean cross-validated habitat suitability.
Figure S17: The temporal distribution of available occurrences for the humpback whale. The histogram shows the number of occurrences within each ten-year bin. The red vertical line differentiates occurrences excluded (before 1980) or included (after 1980) in the analysis. The right plot shows the number of occurrences after 1980 on each calendar day. The majority of occurrences were collected during summer months (particularly in January and February).
Figure S18: Results for Maxent’s jackknifing test for ModelAll (left) and ModelUnique (right) for the humpback whale. Red bars represent the cross-validated mean regularized training gain (a measure of goodness-of-fit) for sub-models calibrated using all predictors except the respective predictor. In contrast, blue bars represent complementary results for models calibrated only with the respective predictor. Error bars represent the standard deviation of the regularized training gain on cross-validation. Grey points show results for the full model, calibrated with no cross-validation. For more information on predictor abbreviations, see Table 1.
Figure S19: Marginal response curves for the humpback whale models. Response curves describe the cloglog prediction changes as each environmental predictor changes, with keeping all other predictors at their average value at training presences. As these response curves are sensitive to the fixed value of other predictors, we also provide the predicted values in the environmental space of the most important three predictors (see Figure S20). Solid blue lines and dashed area represent the mean and standard deviation of the cross-validated response curve of ModelAll, while the red line and dashed area represents ModelUnique. PI represents the permutation importance of the predictor, with predictors with more than 5% permutation importance shown in a larger font. For better understanding, transformed predictors (distance to 1000m isobath, slope, mean summer chlorophyll, annual variability of sea surface height, and annual mean speed) were also back-transformed for visualization. The top green rug shows values at species occurrences; similarly, the bottom rung shows values south of the Polar Front.
Figure S20: Mean habitat suitability of the humpback whale in the environmental space of the three most important environmental predictors. The first row shows combinations at species occurrences, while the second and third rows show predictions from ModelAll and ModelUnique, respectively. In each plot in the second and third row, mean habitat suitability at each respective combination was averaged over all available combinations of other predictors. White areas represent no available environmental combinations in the data. Colours range from blue (low prediction) to red (high prediction). For more information on the predictors acronyms used, see Table 1.
Figure S21: Maps of the 15 environmental predictors used to run the models. For more information on predictor abbreviations, see Table 1.
Figure S22: Pearson correlation coefficient between each pair of environmental predictors used to run the models. Highly correlated predictors were excluded in advance using variance inflation factor (see main text). The maximum value of the correlation coefficient is 0.71. Colours range from red (high negative correlation) to blue (high positive correlation). For more information on predictor abbreviations, see Table 1.
Figure S23: Boxplots comparing values of each environmental predictor south of the Polar Front with corresponding values at species-specific sightings. For more information on predictor abbreviations, see Table 1.
Figure S24: The spatial allocation of blocks into species- and model-specific five-fold cross-validation. Block colour indicates how blocks were distributed into cross-validation folds. ModelAll represents models run using all occurrences, while ModelUnique represents models calibrated after removing duplicated occurrences within each 10×10 km cell. The number below each map represents the size of each block in kilometre. Points represent species presence-only sightings used in this study
Figure S25: Spatiotemporal biases in species observation data. The map to the left shows the number of sightings used in this study (log-scale) per 100×100 km grid. Note the existence of high sampling bias towards the Antarctic Peninsula area and the absence of sightings from the majority of the Weddell Sea (dashed polygon). The plot to the right shows the number of sightings used in this study at each calendar day. There is an inevitable temporal bias in the visual observations data towards the summer months, particularly from the end of December to mid-April.
Figure S26: The seasonal distribution of daily sea ice edge from 2002 to 2019. Here, seasons were determined as three-month intervals from January.
Figure S27: Monthly distribution of daily sea ice edge from 2002 to 2019.
Figure S28: Comparison between distance to sea ice edge (SIE, left plots) or sea ice concentration (SIC, right plots) at species sightings, either spatiotemporally matched with their respective daily distance to SIE or SIC (x-axis) or spatially matched with the mean distance to summer SIE or SIC (y-axis, predictors used in this study). Horizontal and vertical grey lines in the left plots represent the location of SIE. The dashed grey line represents the identity (y=x relationship). It is clear that summarising highly dynamic environmental conditions (mean summer SIC or distance to summer SIE) has highly under- or over-estimated the correct values of SIC and SIE. This can greatly impact the performance of the static models and their inferences in the highly dynamic environment of the SO.
References of Supporting Information
Ainley, D. G., Jongsomjit, D., Ballard, G., Thiele, D., Fraser, W. R., & Tynan, C. T. (2012). Modeling the relationship of Antarctic minke whales to major ocean boundaries. Polar Biology, 35(2), 281-290. Andrews-Goff, V., Bestley, S., Gales, N. J., Laverick, S. M., Paton, D., Polanowski, A. M., . . . Double, M. C. (2018). Humpback whale migrations to Antarctic summer foraging grounds through the southwest Pacific Ocean. Sci Rep, 8(1), 12333. doi:10.1038/s41598-018-30748-4. Beekmans, B. W. P. M., Forcada, J. F., Murphy, E. J., de Baar, H. J. W., Bathmann, U., & Fleming, A. H. (2010). Generalised additive models to investigate environmental drivers of Antarctic minke whale (Balaenoptera bonaerensis) spatial density in austral summer. Journal of Cetacean Research and Management, 11(2), 115-129. Bombosch, A., Zitterbart, D. P., Van Opzeeland, I., Frickenhaus, S., Burkhardt, E., Wisz, M. S., & Boebel, O. (2014). Predictive habitat modelling of humpback (Megaptera novaeangliae) and Antarctic minke (Balaenoptera bonaerensis) whales in the Southern Ocean as a planning tool for seismic surveys. Deep Sea Research Part I: Oceanographic Research Papers, 91, 101-114. Branch, T. A., Stafford, K. M., Palacios, D. M., Allison, C., Bannister, J. L., Burton, C. L. K., . . . Warneke, R. M. (2007). Past and present distribution, densities and movements of blue whales Balaenoptera musculus in the Southern Hemisphere and northern Indian Ocean. Mammal Review, 37(2), 116-175. Dalla Rosa, L., Secchi, E. R., Maia, Y. G., Zerbini, A. N., & Heide-Jørgensen, M. P. (2008). Movements of satellite-monitored humpback whales on their feeding ground along the Antarctic Peninsula. Polar Biology, 31(7), 771-781. Elith, J., et al. (2010). The art of modelling range-shifting species. Methods in Ecology and Evolution 1(4): 330-342. Filun, D., Thomisch, K., Boebel, O., Brey, T., Sirovic, A., Spiesecke, S., & Van Opzeeland, I. (2020). Frozen verses: Antarctic minke whales (Balaenoptera bonaerensis) call predominantly during austral winter. Royal Society Open Science, 7(10). Friedlaender, A. S., Johnston, D. W., Fraser, W. R., Burns, J., Patrick N, H., & Costa, D. P. (2011). Ecological niche modeling of sympatric krill predators around Marguerite Bay, Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 58(13-16), 1729-1740. Herr, H., Kelly, N., Dorschel, B., Huntemann, M., Kock, K. H., Lehnert, L. S., . . . Scheidat, M. (2019). Aerial surveys for Antarctic minke whales (Balaenoptera bonaerensis) reveal sea ice dependent distribution patterns. Ecol Evol, 9(10), 5664-5682. Kasamatsu, F., Ensor, P., Joyce, G. G., & Kimura, N. (2000a). Distribution of minke whales in the Bellingshausen and Amundsen Seas (60 degrees W-120 degrees W), with special reference to environmental/physiographic variables. Fisheries Oceanography, 9(3), 214-223. Kasamatsu, F., Matsuoka, K., & Hakamada, T. (2000b). Interspecific relationships in density among the whale community in the Antarctic. Polar Biology, 23(7), 466-473. Kasamatsu, F. (1988). Distribution of cetacean sightings in the Antarctic: results obtained from the IWC/IDCR minke whale assessment cruises, 1978/79 to 1983/84. International Whaling Commission. Report. 38. United Kingdom. P.449-473. Retrieved from Locarnini, R. A., Mishonov, A. V., Baranova, O. K., Boyer, T. P., Zweng, M. M., Garcia, H. E., . . . Smolyar, I. (2018). World Ocean Atlas 2018, Volume 1: Temperature. A. Mishonov Technical Ed.; NOAA Atlas NESDIS 81, 52 pp. Retrieved from https://www.nodc.noaa.gov/OC5/woa18/pubwoa18.html Murase, H. (2014). Estimation of circumpolar spatial distributions of baleen whales in the Antarctic in the period of the IWC IDCR/SOWER CPII and CPIII (1994 – 2004). Paper SC/65b/IA10 presented to the 65a IWC Scientific Committee, May 2014. 42 pp. Retrieved from https://iwc.int/. Murase, H., Kitakado, T., Hakamada, T., Matsuoka, K., Nishiwaki, S., & Naganobu, M. (2013). Spatial distribution of Antarctic minke whales (Balaenoptera bonaerensis) in relation to spatial distributions of krill in the Ross Sea, Antarctica. Fisheries Oceanography, 22(3), 154-173. Nowacek, D. P., Friedlaender, A. S., Halpin, P. N., Hazen, E. L., Johnston, D. W., Read, A. J., . . . Zhu, Y. (2011). Super-aggregations of krill and humpback whales in Wilhelmina Bay, Antarctic Peninsula. PLoS One, 6(4), e19173. Owen, K., Jenner, K. C. S., Jenner, M. N. M., McCauley, R. D., & Andrews, R. D. (2019). Water temperature correlates with baleen whale foraging behaviour at multiple scales in the Antarctic. Marine and Freshwater Research, 70(1), 19-32. Rankin, S., ljungblad, D., Clark, C., & Kato, H. (2005). Vocalisations of Antarctic blue whales, Balaenoptera musculus intermedia, recorded during the 2001/2002 and 2002/2003 IWC/SOWER circumpolar cruises, Area V, Antarctica. Journal of Cetacean Research and Management, 7(1), 13-20. Riekkola, L., Andrews-Goff, V., Friedlaender, A., Constantine, R., & Zerbini, A. N. (2019). Environmental drivers of humpback whale foraging behavior in the remote Southern Ocean. Journal of Experimental Marine Biology and Ecology, 517, 1-12. Santora, J. A., Schroeder, I. D., & Loeb, V. J. (2014). Spatial assessment of fin whale hotspots and their association with krill within an important Antarctic feeding and fishing ground. Marine Biology, 161(10), 2293-2305. Sathyendranath, S., Grant, M., Brewin, R. J. W., Brockmann, C., Brotas, V., Chuprin, A., . . . Zibordi, G. (2018). ESA Ocean Colour Climate Change Initiative (Ocean_Colour_cci): Version 3.1 Data. Centre for Environmental Data Analysis, 04 July 2018. Schall, E., Thomisch, K., Boebel, O., Gerlach, G., Spiesecke, S., & Van Opzeeland, I. (2020). Large-scale spatial variabilities in the humpback whale acoustic presence in the Atlantic sector of the Southern Ocean. Royal Society Open Science, 7(12). doi:10.1098/rsos.201347 Scheidat, M., Friedlaender, A., Kock, K. H., Lehnert, L., Boebel, O., Roberts, J., & Williams, R. (2011). Cetacean surveys in the Southern Ocean using icebreaker-supported helicopters. Polar Biology, 34(10), 1513-1522. Shabangu, F. W., Yemane, D., Stafford, K. M., Ensor, P., & Findlay, K. P. (2017). Modelling the effects of environmental conditions on the acoustic occurrence and behaviour of Antarctic blue whales. PLoS One, 12(2), e0172705. Širović, A., & Hildebrand, J. A. (2011). Using passive acoustics to model blue whale habitat off the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 58(13-16), 1719-1728. Širović, A., Hildebrand, J. A., Wiggins, S. M., McDonald, M. A., Moore, S. E., & Thiele, D. (2004). Seasonality of blue and fin whale calls and the influence of sea ice in the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 51(17-19), 2327-2344. Spreen, G., Kaleschke, L., & Heygster, G. (2008). Sea ice remote sensing using AMSR-E 89-GHz channels. Journal of Geophysical Research, 113(C2). Thiele, D., Chester, E. T., Moore, S. E., Širovic, A., Hildebrand, J. A., & Friedlaender, A. S. (2004). Seasonal variability in whale encounters in the Western Antarctic Peninsula. Deep Sea Research Part II: Topical Studies in Oceanography, 51(17-19), 2311-2325. Thomisch, K., Boebel, O., Clark, C. W., Hagen, W., Spiesecke, S., Zitterbart, D. P., & Van Opzeeland, I. (2016). Spatio-temporal patterns in acoustic presence and distribution of Antarctic blue whales Balaenoptera musculus intermedia in the Weddell Sea. Endangered Species Research, 30, 239-253. Van Opzeeland, I., Van Parijs, S., Kindermann, L., Burkhardt, E., & Boebel, O. (2013). Calling in the cold: pervasive acoustic presence of humpback whales (Megaptera novaeangliae) in Antarctic coastal waters. PLoS One, 8(9), e73007. Weatherall, P., Marks, K. M., Jakobsson, M., Schmitt, T., Tani, S., Arndt, J. E., . . . Wigley, R. (2015). A new digital bathymetric model of the world’s oceans. Earth and Space Science, 2(8), 331-345. Williams, R., Hedley, S. L., & Hammond, P. S. (2006). Modeling distribution and abundance of Antarctic baleen whales using ships of opportunity. Ecology and Society, 11(1). Williams, R., Kelly, N., Boebel, O., Friedlaender, A. S., Herr, H., Kock, K. H., . . . Brierley, A. S. (2014). Counting whales in a challenging, changing environment. Sci Rep, 4, 4170. Zweng, M. M., Reagan, J. R., Seidov, D., Boyer, T. P., Locarnini, R. A., Garcia, H. E., . . . Smolyar, I. (2018). World Ocean Atlas 2018, Volume 2: Salinity. A. Mishonov Technical Ed.; NOAA Atlas NESDIS 82, 50 pp. Retrieved from https://www.nodc.noaa.gov/OC5/woa18/pubwoa18.html Appendix 1: Data sources accessed via GBIF (https://www.gbif.org/) on 31st July 2018
Administración de Parques Nacionales, Argentina. VERTEBRADOS DE VALOR ESPECIAL EN ÁREAS PROTEGIDAS DE LA ARGENTINA. https://doi.org/10.15468/s5t7po Amaya Montano L, Silva Garrido G (2018). Recopilación de datos e información para el Inventario Nacional de Especies de mamíferos marinos nativos de Chile 2016.. Version 1.4. Ministerio del Medio Ambiente de Chile. https://doi.org/10.15468/hw2skw Antarctic Biodiversity Information Facility (ANTABIF): PolE At Sea Observations of Birds and Marine Mammals during PS 81 (ANT-XXIX/4) https://doi.org/10.15468/eglthy Australian Antarctic Data Centre (2018). APIS - Antarctic Pack Ice Seals 1994-1999, plus historical data from the 1980’s. https://doi.org/10.15468/5j6flc Australian Antarctic Data Centre (2018). Orca observations from the shores of Macquarie Island. https://doi.org/10.15468/pwblnd Australian Antarctic Data Centre (2018). Seabirds of the Southern and South Indian Ocean. https://doi.org/10.15468/tu0dcw Australian Antarctic Data Centre (2020). National Whale and Dolphin Sightings and Strandings Database. https://doi.org/10.15468/f68ga6 Australian Antarctic Data Centre (2020). Whale catches in the Southern Ocean. https://doi.org/10.15468/jfmweg Australian Antarctic Data Centre. Cetacean Sightings Survey and Southern Ocean Cetacean Program - BROKE-West. https://doi.org/10.15468/7pkncj Australian Museum (2021). Australian Museum provider for OZCAM. https://doi.org/10.15468/e7susi British Antarctic Survey. SOMBASE. https://doi.org/10.15468/jjxqmf Chazezau Charlotte N, Duhamel Guy N, Inventaire National du Patrimoine Naturel (2019). Base de données Pecheker. MNHN - DEPARTEMENT MILIEUX PEUPLEMENTS AQUATIQUES. Supplément. Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/36d2hp Chazezau Charlotte N, Duhamel Guy N, Inventaire National du Patrimoine Naturel (2019). Base de données Pecheker (modifiée). Museum National D’Histoire Naturelle - DEPARTEMENT MILIEUX PEUPLEMENTS AQUATIQUES. Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/krj0gz CHERRY Allison N, Inventaire National du Patrimoine Naturel (2019). Données d’observation de Cétacés en Antarctique dans le cadre du Programme SOWER entre 1978 et 2009. Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/erhxyp Claire Garrigue N, Inventaire National du Patrimoine Naturel (2018). Distribution et Abondance des Cétacés en Terre Adélie. CETA 2009-2010. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/nd1v2a Divers N, Inventaire National du Patrimoine Naturel (2020). Inventaire des Mammifères marins de France (métropole et outre-mer). Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/qsofh6 Ekström J (2021). Lund Museum of Zoology (MZLU). GBIF-Sweden. https://doi.org/10.15468/mw39rb García N A, Crespo E A (2015). Colección de Mamíferos Marinos del Centro Nacional Patagónico. Version 1.1. CCT CONICET-CENPAT Centro Científico Tecnológico. https://doi.org/10.15468/i2ycus Kool J (2019). Whale log - observations from ANARE voyages. Australian Antarctic Data Centre. https://doi.org/10.15468/ngkv2s Kool J (2020). Whale Observations from the British, Australian and New Zealand Antarctic Research Expedition (BANZARE) voyages 1929-30 and 1930-31. Australian Antarctic Data Centre. https://doi.org/10.15468/rgdg1u Lanna, Campos, Bassoi. 2008. South American Antarctic MarineBiodiversity Literature. https://doi.org/10.15468/kxnpuq McClennen M, Jenkins J, Uhen M (2017). Paleobiology Database. Paleobiology Database. https://doi.org/10.15468/jfqhiu Museums Victoria (2021). Museums Victoria provider for OZCAM. https://doi.org/10.15468/lp1ctu n/a N, Inventaire National du Patrimoine Naturel (2019). Données d’observations opportunistes de Mammifères marins collectées par les hivernants des TAAF. Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/zlktrw Natural History Museum (2021). Natural History Museum (London) Collection Specimens. https://doi.org/10.5519/0002965 naturgucker.de. naturgucker. https://doi.org/10.15468/uc1apo NIWA (2015). Antarctic Biodiversity Studies 2006 - Ross Sea, Scott Island, and Balleny Islands (TAN0602). Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research, Wellington, New Zealand, 1061 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=tan0602 released on April 17, 2015. https://doi.org/10.15468/9jchw1 Orrell T, Informatics Office (2021). NMNH Extant Specimen Records. Version 1.41. National Museum of Natural History, Smithsonian Institution. https://doi.org/10.15468/hnhrg3 Queen Victoria Museum and Art Gallery (2020). Queen Victoria Museum Art Gallery provider for OZCAM. https://doi.org/10.15468/tedfxn Raymond B, Kool J (2020). Cetacean Sightings Survey and Southern Ocean cetacean program. Australian Antarctic Data Centre. https://doi.org/10.15468/i5bfbf Retana M V, Lewis M N, Dellabianca N A, Raya Rey A, Scioscia G, Torres M (2016): Sightings of marine mammals carried out during oceanographic surveys in the Argentine continental shelf. v1.2. ArOBIS Centro Nacional Patagónico. http://arobis.cenpat-conicet.gob.ar:8081/resource?r=arobis-cenpat-cadic-mammalobs&v=1.2 https://doi.org/10.15468/sauctt Reyes L (2015): Cetacean distribution in the South Atlantic and South Pacific Ocean (AR-OBIS). v1.9. ArOBIS Centro Nacional Patagónico. http://arobis.cenpat-conicet.gob.ar:8081/resource?r=arobis-cetaceans&v=1.9 https://doi.org/10.15468/ytekoo Robert H, Van de Putte A (2018). Marine birds and mammals of the Southern Ocean (a census for the CAML). SCAR - AntOBIS. https://doi.org/10.15468/pldcnz SCAR - AntOBIS: ANTXXIII-8 Birds and Mammals https://doi.org/10.15468/wpsq63 South Australian Museum (2021). South Australian Museum Australia provider for OZCAM. https://doi.org/10.15468/wz4rrh Southwestern Pacific OBIS (2014). Biological observations from the Discovery Investigations 1925-1935. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 33337 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=discovery_reports released on January 23, 2015. https://doi.org/10.15468/qoqbu7 Southwestern Pacific OBIS (2014). British Antarctic (Terra Nova) Expedition, 1910-1913. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 1779 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=terranova released on July 29, 2014 https://doi.org/10.15468/0gsnmz SWPRON (2014). Marine biological observation data from coastal and offshore surveys around New Zealand. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 9092 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=mbis_nz released on January 16, 2018. https://doi.org/10.15468/pzpgop Tasmanian Museum and Art Gallery (2020). Tasmanian Museum and Art Gallery provider for OZCAM. https://doi.org/10.15468/ijp8p9 Teta P, Rodríguez D (2021). Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN). Mammalogy National Collection (MACNMa). Museo Argentino de Ciencias Naturales. https://doi.org/10.15468/ukdxqp Trombone T (2016). AMNH Mammal Collections. American Museum of Natural History. https://doi.org/10.15468/wu3poe Ueda K (2021). iNaturalist Research-grade Observations. iNaturalist.org. https://doi.org/10.15468/ab3s5x UMS PatriNat (OFB-CNRS-MNHN), Paris (2019). Données de Mammifères marins dans les TAAF et autres territoires issues de la bibliographies. Compte cardobs 1007 (Trudelle). Version 1.1. https://doi.org/10.15468/dpxcgg VALLEE Dominique N, DELORD Karine N, Inventaire National du Patrimoine Naturel (2019). Observations de mammifères marins issues des campagnes dans les Terres Australes et Antarctiques Françaises (programme 109 Institut Polaire Français - IPEV). Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/nutvhu VAN CANNEYT Olivier N, DABIN Willy N, Inventaire National du Patrimoine Naturel (2019). Observations de mammifères marins du Réseau National d’Echouages en Outre-Mer. Version 1.1. UMS PatriNat (OFB-CNRS-MNHN), Paris. https://doi.org/10.15468/qtvylt Vishnyakova K (2016): Antractic whale observation on a platform of opportunity abroad krill fishing vessel “Dalmor II”, April - July 2011.. v1.9. Ukrainian Scientific Centre of Ecology of the Sea (UkrSCES). http://gp.sea.gov.ua:8082/ipt/resource?r=whale_observation_ccamlr_ukrsces&v=1.9 https://doi.org/10.15468/khjvhb Western Australian Museum (2019). Western Australian Museum provider for OZCAM. https://doi.org/10.15468/5qt0dm Woehler E (2020): RV Investigator Voyage IN2017_V02 Seabird Observations, Australia (2017). v1.9. CSIRO National Collections and Marine Infrastructure (NCMI) Information and Data Centre (IDC). https://www.marine.csiro.au/ipt/resource?r=in2017_v02_wov&v=1.9 https://doi.org/10.15468/swdgpq Appendix 2: Data sources accessed via iOBIS (https://obis.org/) on 26th July 2018
ANTXXIII-8 Birds and Mammals. https://obis.org/dataset/d815b624-43e6-4e3e-9136-094353e776f8 APIS - Antarctic Pack Ice Seals 1994-1999, plus historical data from the 1980s. https://obis.org/dataset/7e92433a-d086-4527-ab2b-3af86bcbd083 Australian Antarctic Data Centre (2018). Whale catches in the Southern Ocean. https://obis.org/dataset/54bf169c-13f6-44cd-8551-06b999c994a1 Australian Antarctic Data Centre. Whale log - observations from ANARE voyages. https://obis.org/dataset/8c844caa-0559-4482-b506-7ab97915376a CAML bird and mammal census in the Southern Ocean. I.R.Sc.N.B. / I.P.F. supported by “Fond Léopold III”. https://obis.org/dataset/7350daa3-7c45-4f0f-b897-32b2a2b9c241 Curtice, C., A. Friedlaender, P. Halpin, D. Johnston, H. Ducklow and N. Gales. 2015. LTER Antarctic satellite telemetry of humpback whales. https://obis.org/dataset/b9a2fd8a-91a1-4285-8ea0-7c8991e811b8 Dabin, W. 2020. Observatoire Pelagis - Reseau National Echouage (French stranding network) strandings 1934-2018. https://obis.org/dataset/553e813b-993b-46f4-b42e-d3a5bf064572 Hyrenbach, D. 2007. SIO Marine Bird and Mammal Survey 2003. https://obis.org/dataset/84cfefce-ded2-4a7f-bb46-6501d724ce0c Hyrenbach, D. 2007. SIO Marine Bird and Mammal Survey 2004. https://obis.org/dataset/a5edf42e-7768-4d1a-8df1-e13fd5d7c8c5 iziko South African Museum - Fish Collection. https://obis.org/dataset/8a1ae661-e911-4967-bc06-1168fc5f2d89 Jayasankar, P. 2008. Cetaceans in the Southern Indian Ocean 2004. https://obis.org/dataset/766fd9a5-2fec-4916-b8b0-7d92bbb1d99b Johnston TH. (2000) Whale and Seal Observations 1929-31 and 1930-31 Pp 30-65. in Shaughnessy, P.D. (ed.) 2000 Antarctic seals, whales and dolphins of the early twentieth century: Marine mammals of the Australasian Antarctic Expedition 1911-14 (AAE) and the British, Australian and New Zealand Antarctic Research Expedition 1929-31 (BANZARE), ANARE Reports 142, 172pp. https://obis.org/dataset/5082de5d-7e3a-4c5f-9da6-b0d4952b7652 Lanna, Campos, Bassoi. 2008. South American Antarctic MarineBiodiversity Literature. https://portal.obis.org/dataset/6af80242-edfd-4dd2-b957-8e715789e479 Maughan, B. and K. Arnold. 2010. UK Royal Navy Marine Mammal Observations. https://obis.org/dataset/5a804d4e-7393-46ff-971c-0beceba02086 Museums Victoria Mammalogy Collection - Marine records. https://obis.org/dataset/4550423a-25fc-4028-ad91-e8f63ecbbc8b National Whale and Dolphin Sightings and Strandings Database. https://obis.org/dataset/0b91d8f6-a50e-44e4-b313-0df7f91f7483 NatureWatch NZ (2016). Marine species citizen-science observations from NatureWatch NZ. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 13030 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=naturewatchnz released on March 26, 2017. https://obis.org/dataset/23c15f33-94a8-4e3e-bb76-5d5b0bf203f8 NIWA (2015). Antarctic Biodiversity Studies 2006 - Ross Sea, Scott Island, and Balleny Islands (TAN0602). Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research, Wellington, New Zealand, 1061 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=tan0602 released on April 17, 2015. https://obis.org/dataset/33b5f094-38b0-4ad2-82d8-1e57f9bc291c PolE At Sea Observations of Birds and Marine Mammals during PS 81 (ANT-XXIX/4). https://obis.org/dataset/b3bdb08f-0f29-4a81-b85e-90e114a8fda6 Retana M V, Lewis M N, Dellabianca N A, Raya Rey A, Scioscia G, Torres M (2016): Sightings of marine mammals carried out during oceanographic surveys in the Argentine continental shelf. v1.2. ArOBIS Centro Nacional Patagónico. Dataset/Occurrence. http://arobis.cenpat-conicet.gob.ar:8081/resource?r=arobis-cenpat-cadic-mammalobs&v=1.2 https://obis.org/dataset/4c50edb9-a5ff-48e4-af3b-ce50c28cd304 Reyes L (2015): Cetacean distribution in the South Atlantic and South Pacific Ocean (AR-OBIS). v1.9. ArOBIS Centro Nacional Patagónico. Dataset/Occurrence. http://arobis.cenpat-conicet.gob.ar:8081/resource?r=arobis-cetaceans&v=1.9 https://obis.org/dataset/72bb39c7-7bb4-435c-affc-1aa66a5b2a5b Seabirds of the Southern and South Indian Ocean. https://obis.org/dataset/832a055e-49ee-40bd-b138-c29cc07606d7 South Australian Museum Mammology Collection. https://obis.org/dataset/105b3c12-a0c1-497e-bc61-4d8a09c30a69 Southwestern Pacific OBIS (2014). Biological observations from the Discovery Investigations 1925-1935. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 33337 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=discovery_reports released on January 23, 2015. https://obis.org/dataset/364e5a12-8028-4329-be50-fc548c2d6ce4 Southwestern Pacific OBIS (2014). British Antarctic (Terra Nova) Expedition, 1910-1913. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 1779 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=terranova released on July 29, 2014. https://portal.obis.org/dataset/159421b6-a764-4af1-9cc3-bc652dbeb12e SWPRON (2014). Marine biological observation data from coastal and offshore surveys around New Zealand. Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand, 9092 records, Online http://nzobisipt.niwa.co.nz/resource.do?r=mbis_nz released on January 16, 2018. https://obis.org/dataset/b339f72f-eb69-40c5-b60c-1a37aaffdfb7 Thiele, D. (2005) Cetacean Sightings Survey and Southern Ocean cetacean program. https://obis.org/dataset/7ca781bf-163f-4202-86ea-cb9a243d878a Vishnyakova K (2016): Antractic whale observation on a platform of opportunity abroad krill fishing vessel “Dalmor II”, April - July 2011.. v1.9. Ukrainian Scientific Centre of Ecology of the Sea (UkrSCES). Dataset/Occurrence. http://gp.sea.gov.ua:8082/ipt/resource?r=whale_observation_ccamlr_ukrsces&v=1.9 https://portal.obis.org/dataset/e6513ed4-0c07-400c-8ec4-9b54b664cdb8 Watts, D.J. (2006) Orca observations on Macquarie Island. https://obis.org/dataset/1b37891a-9d12-4f54-a4fc-6d5dd5dd92b3 Webmaster O (2020): Tasmanian Museum and Art Gallery provider for OZCAM - marine records. v1.7. CSIRO Oceans and Atmosphere. Dataset/Occurrence. https://www.marine.csiro.au/ipt/resource?r=tmag_marine&v=1.7 https://obis.org/dataset/8faf0e94-1c0e-479d-942c-7f21a4c0a5b4 Woolmer, G. 2013. Historical distribution of whales shown by logbook records 1785-1913. https://obis.org/dataset/a9848680-b804-455a-b6b5-2c5c1d0dbcde
Appendix 3: Data accessed via OBIS-SEAMAP (http://seamap.env.duke.edu/) on 22nd August 2018
- MV Mammals. http://seamap.env.duke.edu/dataset/103150076
- SAM Mammals. http://seamap.env.duke.edu/dataset/103150083 Curtice, C., A. Friedlaender, P. Halpin, D. Johnston, H. Ducklow and N. Gales. 2015. LTER Antarctic satellite telemetry of humpback whales. http://seamap.env.duke.edu/dataset/1261 Dabin, W. 2020. Observatoire Pelagis - Reseau National Echouage (French stranding network) strandings 1934-2018. http://seamap.env.duke.edu/dataset/1406 Danis, B. 2020. ANTXXIII-8 Birds and Mammals. http://seamap.env.duke.edu/dataset/103151600 — https://obis.org/dataset/d815b624-43e6-4e3e-9136-094353e776f8 Gorton, R and D. Thiele. 2020. Cetacean Sightings Survey and Southern Ocean cetacean program. http://seamap.env.duke.edu/dataset/103150150 — https://obis.org/dataset/2ab04179-c3e5-4754-9df5-65d3b3e0dbcd Happywhale. 2020. Happywhale - Minke Whale in South Pacific Ocean. http://seamap.env.duke.edu/dataset/1744 Happywhale. 2020. Happywhale - Southern Bottlenose Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1736 Happywhale. 2021. Happywhale - Arnoux’’s Beaked Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1731 Happywhale. 2021. Happywhale - Blue Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1759 Happywhale. 2021. Happywhale - Blue Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1760 Happywhale. 2021. Happywhale - Fin Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1752 Happywhale. 2021. Happywhale - Fin Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1753 Happywhale. 2021. Happywhale - Humpback Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1766 Happywhale. 2021. Happywhale - Humpback Whale in South Pacific Ocean. http://seamap.env.duke.edu/dataset/1768 Happywhale. 2021. Happywhale - Humpback Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1767 Happywhale. 2021. Happywhale - Killer Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1719 Happywhale. 2021. Happywhale - Killer Whale in South Pacific Ocean. http://seamap.env.duke.edu/dataset/1721 Happywhale. 2021. Happywhale - Killer Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1720 Happywhale. 2021. Happywhale - Long-finned Pilot Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1781 Happywhale. 2021. Happywhale - Long-finned Pilot Whale in South Pacific Ocean. http://seamap.env.duke.edu/dataset/1782 Happywhale. 2021. Happywhale - Minke Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1743 Happywhale. 2021. Happywhale - Sei Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1747 Happywhale. 2021. Happywhale - Sei Whale in South Pacific Ocean. http://seamap.env.duke.edu/dataset/1748 Happywhale. 2021. Happywhale - Southern Bottlenose Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1735 Happywhale. 2021. Happywhale - Southern Right Whale in South Atlantic Ocean. http://seamap.env.duke.edu/dataset/1783 Happywhale. 2021. Happywhale - Southern Right Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1784 Happywhale. 2021. Happywhale - Sperm Whale in Southern Ocean. http://seamap.env.duke.edu/dataset/1729 Hyrenbach, D. 2007. SIO Marine Bird and Mammal Survey 2003. http://seamap.env.duke.edu/dataset/95 Hyrenbach, D. 2007. SIO Marine Bird and Mammal Survey 2004. http://seamap.env.duke.edu/dataset/227 Johnston TH. (2000) Whale and Seal Observations 1929-31 and 1930-31 Pp 30-65. in Shaughnessy, P.D. (ed.) 2000 Antarctic seals, whales and dolphins of the early twentieth century: Marine mammals of the Australasian Antarctic Expedition 1911-14 (AAE) and the British, Australian and New Zealand Antarctic Research Expedition 1929-31 (BANZARE), ANARE Reports 142, 172pp. See Metadata record http://data.aad.gov.au/aadc/metadata/metadata_redirect.cfm?md=AMD/AU/ASAC_140. Maughan, B. and K. Arnold. 2010. UK Royal Navy Marine Mammal Observations. http://seamap.env.duke.edu/dataset/64 Obis secretariat. 2020. Antarctic Pack Ice Seals 1994-1999, plus historical data from the 1980’s. http://seamap.env.duke.edu/dataset/103150146 — https://obis.org/dataset/de164752-b2a3-4857-b73b-04c00c0c801e Obis secretariat. 2020. National Whale and Dolphin Sightings and Strandings Database. http://seamap.env.duke.edu/dataset/103150567 — https://obis.org/dataset/5d6e3670-3455-434b-b2fe-3d50f144b51a Reyes, L. 2011. Cetacean distribution in the South Atlantic and South Pacific Ocean (AR-OBIS). http://seamap.env.duke.edu/dataset/103150519. Robertson, H. 2020. iziko South African Museum - Marine Mammal Collection. http://seamap.env.duke.edu/dataset/103150130 — https://obis.org/dataset/2e471257-d3af-40f7-ba72-fccdb460fc2b Van De Putte, A. 2011. Marine birds and mammals of the Southern Ocean (a census for the CAML). http://seamap.env.duke.edu/dataset/103151589. Van De Putte, A., W. Decock and A. Anunciante. 2011. South American Antarctic Marine Biodiversity Literature. http://seamap.env.duke.edu/dataset/103151611. Watts, D. (2009) Whale log - observations from ANARE voyages, Australian Antarctic Data Centre - CAASM Metadata (http://data.aad.gov.au/aadc/metadata/ Watts, D. 2020. Orca observations on Macquarie Island. http://seamap.env.duke.edu/dataset/103150149 — https://obis.org/dataset/f54e9964-2d7f-4f6e-8977-de46d01c3cfd Watts, D. 2020. Whale catches in the Southern Ocean. http://seamap.env.duke.edu/dataset/103150022 — https://obis.org/dataset/a4d4e3f2-7626-49bb-b85b-16ed83105909 Woolmer, G. 2013. Historical distribution of whales shown by logbook records 1785-1913. http://seamap.env.duke.edu/dataset/885 Appendix 4: Reference list of Polarstern data used
Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXV/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.728270 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXII/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729027 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXII/4. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729028 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729030 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729031 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/4. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729032 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/5. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729033 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/6. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729034 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/7. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729035 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/8. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729036 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIII/9. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729037 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIV/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729040 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIV/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729041 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXIV/4. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729042 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXV/3 (LOHAFEX. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729044 Burkhardt, E. (2009): Whale sightings during POLARSTERN cruise ANT-XXV/4. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.729045 Burkhardt, E. (2011): Whale sightings during POLARSTERN cruise ANT-XXVI/1. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.760335 Burkhardt, E. (2011): Whale sightings during POLARSTERN cruise ANT-XXVI/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.760336 Burkhardt, E. (2011): Whale sightings during POLARSTERN cruise ANT-XXVI/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.760337 Burkhardt, E. (2011): Whale sightings during POLARSTERN cruise ANT-XXVII/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.760340 Burkhardt, E. (2012): Whale sightings during POLARSTERN cruise ANT-XXVII/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.783806 Burkhardt, E. (2013): Whale sightings during POLARSTERN cruise ANT-XXVIII/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819861 Burkhardt, E. (2013): Whale sightings during POLARSTERN cruise ANT-XXVIII/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819862 Burkhardt, E. (2013): Whale sightings during POLARSTERN cruise ANT-XXVIII/4. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819863 Burkhardt, E. (2013): Whale sightings during POLARSTERN cruise ANT-XXVIII/5. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819864 Burkhardt, E. (2013): Whale sightings during POLARSTERN cruise ANT-XXIX/2. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.819866 Burkhardt, E. (2014): Whale sightings during POLARSTERN cruise ANT-XXIX/3. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.840382 Burkhardt, E. (2018): Whale sightings during POLARSTERN cruise PS103. Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA, https://doi.org/10.1594/PANGAEA.895984 Appendix 5: Reference list of PANGAEA (https://www.pangaea.de/) data used
Herr, H.; Lehnert, L. S.; Siebert, U. (2018): Aerial cetacean survey Southern Ocean 2010/2011. PANGAEA, https://doi.org/10.1594/PANGAEA.894934 Herr, H.; Lehnert, L. S.; Siebert, U. (2018): Ship based cetacean survey Southern Ocean 2010/2011. PANGAEA, https://doi.org/10.1594/PANGAEA.894910 Herr, H.; Lehnert, L. S.; Siebert, U. (2018): Ship based cetacean survey Southern Ocean 2011/2012. PANGAEA, https://doi.org/10.1594/PANGAEA.894909 Herr, H.; Siebert, U. (2018): Aerial cetacean survey Southern Ocean 2012/2013. PANGAEA, https://doi.org/10.1594/PANGAEA.894914 Herr, H.; Viquerat, S.; Siebert, U. (2018): Aerial cetacean survey Southern Ocean 2014/2015. PANGAEA, https://doi.org/10.1594/PANGAEA.894938 Herr, H.; Viquerat, S.; Siebert, U. (2018): Ship based cetacean survey Southern Ocean 2014/2015. PANGAEA, https://doi.org/10.1594/PANGAEA.894873 Herr, H.; Viquerat, S.; Siebert, U. (2018): Ship based cetacean survey Southern Ocean 2016. PANGAEA, https://doi.org/10.1594/PANGAEA.894912 Scheidat, M.; Herr, H. (2018): Aerial cetacean survey Southern Ocean 2006/2007. PANGAEA, https://doi.org/10.1594/PANGAEA.894937 Scheidat, M.; Herr, H.; Siebert, U. (2018): Aerial cetacean survey Southern Ocean 2008/2009. PANGAEA, https://doi.org/10.1594/PANGAEA.894936 Scheidat, M.; Herr, H.; Siebert, U. (2018): Ship based cetacean survey Southern Ocean 2008/2009. PANGAEA, https://doi.org/10.1594/PANGAEA.894911
Acknowledgments: This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant number 2817HS004. We thank Andy Traumüller for helping in environmental variables preparation and all data providers for making their observation data publicly available, especially captains and nautical officers onboard Polarstern. The manuscript was improved by comments of Alaaeldin Soultan. We appreciate suggestions from Carsten Dormann and David Warton on how background locations should be sampled for presence-only dynamic SDMs. This work was partially performed on the HPC computer facility of the Alfred Wegener Institute. We thank Natalja Rakowsky for technical support on the HPC computers.
Biosketch: The authors are members of the ocean acoustics group at the Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research. They are interested in exploring the distribution and behaviour of marine mammals and their underwater acoustic environment in the polar oceans using long-term (multi-year) visual observations and passive acoustic recordings. They aim at providing reliable information on year-round distributions of marine mammals in polar oceans to inform conservation, management, and science. They are particularly interested in using static and dynamic species distribution models in polar areas using visual and PAM data. Author contributions: All authors conceived the research; AE-G performed data curation, planned and conducted the analyses; AE-G wrote the first draft, with all authors contributing to the writing of the final manuscript.